University Animal Care Committee Standard Operating Procedure
Document No: 7.12
Subject: Rodent Tail Vein Injections
Date Issued: July 7, 2011
Revision: 3
Location: Queen’s University
Responsibility: Principal Investigators (PI), Research Staff, Veterinary Staff
Purpose: The purpose of this Standard Operating Procedure (SOP) is to describe the procedure of intravenous substance administration via the tail vein.
1. Introduction and Definitions:
The lateral tail vein is a commonly used injection site in mice and rats. The procedure causes only minimal or transient pain and distress and does not require anesthesia in the mouse.
2. Materials:
*All drugs, fluids, suture material and autoclaved items must not be used if passed their expiry date*
MOUSE
- EMLA cream*
- 26-30g needle (use the smallest gauge possible to allow accurate infusion and to accommodate particle size and viscosity)
- Appropriate sized syringe (usually 1ml)
- Injectable solution
- Mouse restrainer
- Heat lamp or vessel with warm water
- 70% isopropyl alcohol
- 2” x 2” gauze
RAT
- EMLA cream*
- 25-27g needle (use the smallest gauge possible to allow accurate infusion and to accommodate particle size and viscosity)
- Appropriate sized syringe (usually 1ml)
- Injectable solution
- Rat restrainer
- Heat lamp or vessel with warm water
- 70% Isopropyl alcohol
- 2” x 2” gauze
- Anesthetic, if required
*EMLA cream requires a minimum 15 minute absorption time post application (species and site dependent). It is strongly recommended to use this topical anesthetic prior to any injection (particularly in the case of novice handlers), however this step may be waived if it contributes to an animal’s stress level and/or is impractical in its application.
3. Procedures
Each and every injection requires a new sterile syringe and a new sterile needle.
The injectable solution:
- The volume to be injected may not exceed 10% of the rodent’s total blood volume (mouse = 72 ml/kg; rat = 64 ml/kg).
- If possible, warm the solution to room temperature immediately prior to injection.
- Substances to be injected must be sterile as contamination can lead to infection or irritation of the injection site. Sterilize solutions by autoclaving or microfiltration and use aseptic technique for injection.
- Remove animal from home cage and apply EMLA cream. Return to home cage for 15 minutes (allows topical anaesthetic to take affect).
- Using sterile technique, load the syringes. Prepare one syringe and one needle per infusion.
- Carefully transfer animal from the home cage to the restrainer.
- Induce peripheral vasodilation using a heat lamp focused on the tail held approximately 20cm away. Use caution to prevent overheating/burning. Alternatively, the tail may be immersed in a warm water bath for 20-30 seconds.
- RATS: Mature rats often require cleaning of the tail with chlorhexidine. With dampened gauze, scrub gently to remove scale and dirt build-up. This step also contributes towards softening the epidermis. If using anesthetic, ensure eye lubricant is instilled.
- Locate the lateral tail veins by elevating the tip of the tail slightly and rotating gently. The veins are located superficially just under the skin.
- In mice with a white background the veins are often visible, however, with mice on a black background and oftentimes rats, the veins are not obvious and may be found only through anatomical landmarking. An additional light source may help visualize the vessel.
- Use forefinger and middle finger on your holding hand to “clamp” the base of the tail of the animal. This helps to stop venous return without stopping the arterial flow.
- Swab the injection site with 70% isopropyl alcohol (wipe in a downwards motion, from base to tip)
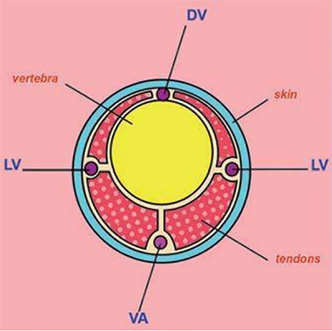
Diagram of a transverse sectional view of a rat tail showing the dorsal vein (DV), lateral veins (LV), and ventral artery (VA). (Modified image reprinted from The Laboratory Rat, G.J. Krinke (Ed.), pp. 491, Copyright 2000)
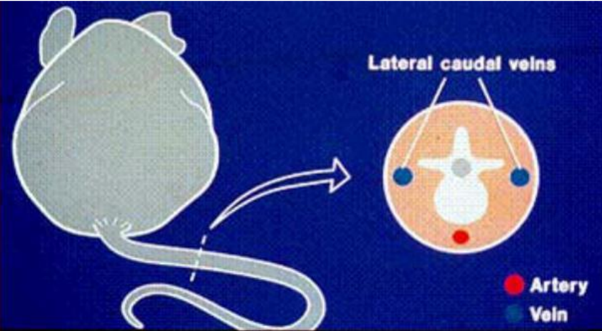
Diagram of a transverse sectional view of mouse tail lateral veins and ventral artery. Kathryn Flynn, NIH - DVR - SoBran
- MICE: Hold the tail under slight tension. With the bevel of the needle facing upward and the needle almost parallel to the vein, slide the needle about 2-3mm into the vessel. The initial puncture should be within the caudal 1/3 of the tail. If the needle is in the vein you may see a flash of blood in the hub of the needle, although this is not always the case. Do not aspirate as it will cause the vein to collapse.
- RATS: Hold the tail under slight tension. With the bevel of the needle facing upward and the needle almost parallel to the vein, slide the needle about 3-5mm into the vessel. The initial puncture should be within the caudal 1/3 of the tail. Confirm the location by gently pulling the plunger; if the needle is in the vein you should see a flash of blood in the hub of the needle, although this is not always the case. Do not aspirate as it will cause the vein to collapse.
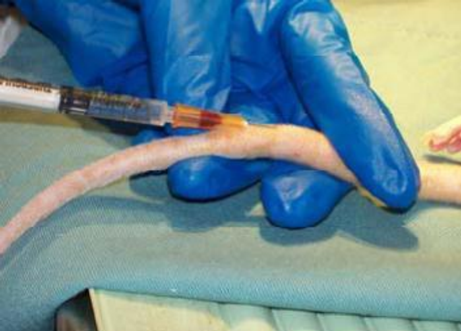
Anaesthetized rat
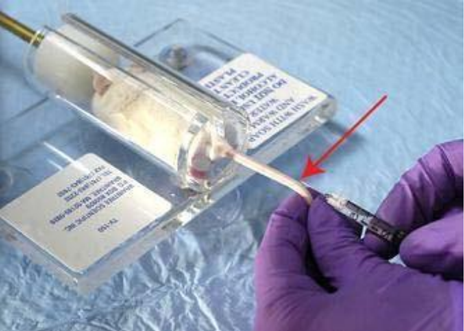
Non-anaesthetized mouse in restrainer
- Push the plunger in slowly and steadily with your finger until the required volume has been injected. If the needle is in the vein, there will be no resistance. If the needle is not in the vein, the solution will be infused perivascular and cause skin blanching or a subcutaneous bleb. Accurate placement can also be confirmed when the vessel clears as the compound is administered and the fluid temporarily replaces the blood.
- A second attempt may be performed by removing the needle and attempting a site on the same vessel in a more cranial location on the tail. Additional attempts can be made on the contra- lateral vein, however limit the number of puncture attempts to three. After three unsuccessful attempts, request the assistance of another trained person.
- Administer infusion at a constant flow rate.
- Do not inject into inflamed or damaged tissue.
- Remove the needle from the vein and apply light pressure to the puncture site with gauze.
- Remove the mouse from the restrainer and place in its home cage.
- Monitor the animal for 5-10 minutes to ensure hemostasis.
| Date | New Version |
|---|---|
| 11/26/2015 | |
| 02/28/2019 | |
| 02/28/2022 |
