Haldane's Rule
 |
"When
in the F1 offspring of two different animal races one sex is absent, rare, or
sterile, that sex is the heterozygous [heterogametic] sex." |
|
J.B.S. Haldane (1922)
Sex-ratio and unisexual sterility in hybrid animals. J.
Genetics 12, 101-109.
|
Introduction
Two Schools of Thought
The Chromosomal Viewpoint
Naveira & Masides' "Unexpected" Result
Paper
on Haldane's Rule (2000)
Who is the Lady?
Introduction
When
sex is chromosomally determined, the sex of an organism is determined by the sex
chromosomes it inherits from its parents.
- The "heterogametic sex"
is the sex with two different sex chromosomes (e.g. X and Y).
- The "homogametic sex"
is the sex with one type of sex chromosome (e.g. X and X).
|
Thus
in humans there are two types of spermatozoa, X-spermatozoa and Y-spermatozoa.
If these are produced in equal quantitities by the heterogametic sex (the normal
situation) then, on average, half the
children will be male (XY), and half
the children will be female (XX). |
 |
|
The two types
of human spermatozoa at surface of ovum
|
If, within a species,
there is a slight tendency for two lines (varieties, races) to diverge as separate species,
then copulation is unimpaired, and healthy children are produced, but some
of those children may be sterile (unable themselves to produce children).
Haldane noted that when the offspring ("F1 hybrid") of a cross between a
male parent from one line and a female parent from the other line is sterile
although otherwise healthy, it will tend to be of the heterogametic sex. This is Haldane's
rule for hybrid sterility. If some gametes are produce by the
F1 hybrid they
will be of both types. Thus, among its
offspring, albeit reduced in number, the usual 50% : 50% male/female ratio is
not disturbed. There is no preferential production of one sex.
If,
within a species, the divergence between the two parental lines becomes sufficient to
generate genic differences, but
copulation is unaffected, then parental gene products may fail to cooperate during development of the
fertilized ovum, resulting in hybrid inviability. Remarkably, here often
the 50% : 50% male/female ratio is disturbed. There is preferential production of one
sex, which is usually the homogametic
sex. The heterogametic sex is absent or rare. This is Haldane's
rule for hybrid inviability.
If
speciation occurs within one geographic region, the first stage of the
speciation process is complete when all hybrids, although viable, are fully sterile
(hybrid sterility). As species differentiation further progresses, parental genic
incompatibilities arise and hybrids fail to be formed. They
fail to develop as embryos or to reach adult maturity (hybrid inviability).
So the question of their sterility does not arise. There are no mature
offspring to be sterile!
As species differentiation further progresses,
parental genic differentiations generate anatomical, physiological or
psychological differences that result in mating incompatibilities. The "postzygotic barriers" (hybrid sterility and hybrid inviability), are replaced by
"prezygotic barriers" (e.g. mating
incompatibilities). So the questions of offspring inviability and
sterility do not arise. There are no young offspring to be inviable, or
mature offspring to be sterile!
|
Two Schools of
Thought Regarding the Form of Hybrid Sterility Associated with Most Speciation Events
|
CHROMOSOMAL |
|
GENIC |
| Romanes
1886
Bateson 1904
Kholodkovskii 1912
Darlington 1932
Gulick 1932
Goldschmidt 1940
White 1978
King 1993 |
|
Dobzhansky 1934
[MAINSTREAM OPINION]
Coyne & Orr 1998 |
|
"In
1912 [Petersburg
entomologist Nicolai] Kholodkovskii
suggested that the origin of the two biological species of Chermes
--
may have been based on their different number of
chromosomes which made them incapable of interbreeding -- . Iurii
Filipchenko - Dobzhansky's mentor in genetics - supported this idea (Filipchenko
1916)"
Krementsov (1994) Dobzhansky and Russian
entomology. The origin of his ideas on species and speciation. The
Evolution of Theodosius Dobzhansky (Princeton Univ. Press) |
"A New
Paradigm"?
"Two of the strongest
patterns in evolutionary biology, Haldane's rule and the large effect of the sex
chromosomes on postzygotic isolation, still lack wholly convincing explanations. It
seems possible that some simple explanation for both phenomena has eluded everyone."
Jerry A. Coyne
(1992) Genetics and speciation. Nature
355, 511-515.
"The concept of genic incompatibility as a
cause of postzygotic isolation -- forms an integral part of most hypotheses, either
implicitly or explicitly."
Cathy C. Laurie (1997)
The weaker
sex is heterogametic: 75 years of Haldane's rule. Genetics
147, 937-951.
"What makes hybrid male sterility of
great current interest is the increasing evidence that the building blocks of this
isolating barrier may be radically different from what we had come to believe
-- . Most
probably, they [the building blocks] are not a more or less abundant collection of genes with
individually detectable effects on hybrid fertility (multigenic basis), but a large number
of interacting gene sets, made out of minor factors whose individual
--
[action] has virtually no effect (polygenic basis). It is clear that a new paradigm is emerging, which will force
us to, first, revise many conclusions of past studies that had gathered almost unanimous
agreement, and second, try a completely different experimental approach to unveil the
genetic basis of hybrid male sterility"
Horacio F. Naveira & Xulio R. Maside
(1998) The genetics of hybrid male sterility in Drosophila. In: Enless Forms: Species and Speciation.
Oxford University Press. pp. 330-338.
For more on History of Haldane's Rule
Click Here |
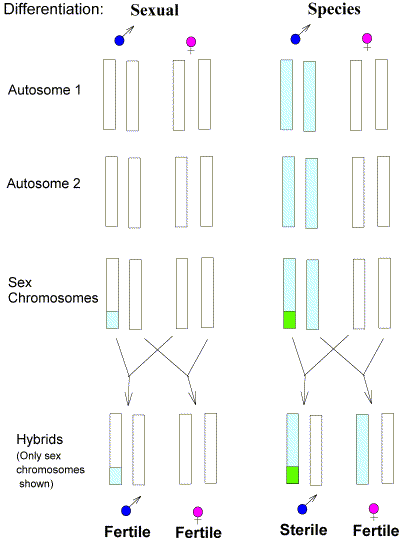
Haldane's Rule from the Chromosomal Viewpoint
|
Bateson's Meditations
"Whenever sexual organisms breeding
together produce a mixture of forms, there is, -- prima
facie reason to suspect that the mixture is due to differentiation of
germs. The
most familiar case is sex itself. A population consisting of males
and females has so many features in common with the differentiating offspring resulting
from the segregation of characters among the germ-cells of cross-bred organisms that it is
impossible to avoid the suspicion that the two phenomena are similar in causation.
A categorical proof of this conclusion would make a remarkable advance in biology."
William Bateson (1906) Application to
the Balfour Fund for a research grant . [For earlier thoughts of Bateson
(Click Here)]
"We may feel fairly sure that the distinction
between the sexes depends on the presence in one or other of them of an unpaired
factor -- .The results of genetic research are so bewilderingly novel
-- . In all the
discussions of the stability and fitness of species who ever contemplated the possibility
of a wild species having one of its sexes permanently hybrid [XY or WZ
chromosomes]? -- Who would have supposed it possible that the pollen cells
of a plant could be all of one type, and it egg cells of two types? Yet Miss Saunders'
experiments have provided definite proof that this is the condition of certain [dioecious] stocks [where
the female is the heterogametic sex] -- ."
William Bateson (1908)
in 'The Methods and Scope of Genetics'. Inaugural Lecture at the University of
Cambridge.
"We have now to admit the further conception that between the male and
female sides of the same plant these ingredients may be quite differently apportioned, and
that the genetical composition of each may be so distinct that the systematist might
without extravagance recognize them as distinct specifically [mistake
the different sexes as distinct species]. If then our plant may by
appropriate treatment be made to give off two distinct forms, why
is not that phenomenon a true instance of Darwin's origin of species?"
William Bateson (1922)
address in Toronto on 'Evolutionary Faith and Modern Doubts'.
Goldschmidt's Meditations
"If
-- genic balance does not account for the facts, we must turn -- to an
effect of the chromosome as a whole, an effect of the whole chromosome
with its specific serial pattern upon the whole reaction system of the
plant. No genic balance is needed and, as for that, no genes either, to
understand the reported facts, as soon as we have decided to assume that a
chromosome of a definite structural pattern is acting as a unit." (pp.
233)
"Sexual
differences within a species may be of such a nature that, if found
distributed among different organisms, they would provide a basis for
classification into different species, families, or even higher
categories. -- Two forms found in nature, which showed morphological
differences of such a degree as that existing between male and female
insects -- would never be considered as belonging to the same species, or
even genus. -- In the sexual differences we have, then, two completely
different reaction systems in which the sum total of all the differences
is determined by a single genetic differential. -- The genetics of sex
determination ought, therefore, to furnish information on how a completely
different reaction system may be evolved on the basis of existing --
genetical agencies." (pp. 234)
"It
is not this or that gene or array of genes which is acting to produce the
extreme morphological differences of the sexes, but rather the typical
serial pattern within the X-chromosome, or definite parts thereof. The
chromosome as a whole is the agent, controlling whole reaction systems (as
opposed to individual traits). The features which are assumed by many
geneticists to prevent a scattering of individual sex genes by crossing
over (one X in heterogametic sex, or inert Y) actually prevent major
changes in the pattern within the chromosome as a whole. -- I must
emphasize that such a conception offers mental difficulties to those
steeped in the classical theory of the gene." (pp.
236)
Richard Goldschmidt.
The Material Basis of Evolution (1940) Yale University
Press.
|
Hybrid Inviability
|
The hybrid inviability
occurring by the mechanism proposed in 1995 (dosage compensation; Click Here),
and by some other mechanisms, would not
be expected in "species" of genera with homomorphic
sex chromosomes (i.e. the sex-determining chromosomes appear similar).
|
|
Mosquitos of the genus Aedes fit
this criterion. Presgrave and Orr (1998; Science
282, 952-954) found for crosses between Aedes "incipient species" that Haldane's rule was associated with
male hybrid sterility, not
inviability.
|
 |
|
Plants with separate sexes (dioecious
plants) and with homomorphic sex chromosomes also follow Haldane's rule
regarding male sterility, not inviability (see below). |
|
Hybrid Sterility
Hybrid
sterility, due to failure of meiotic pairing, might reflect initial (C+G)%
differences between the regions concerned with sexual differentiation (i.e. differences in
base composition would be the "building blocks"
referred to by Naveira and Maside, above). This hypothesis differs from the main line of
chromosomal hypotheses for hybrid sterility in that the chromosomal differentiations are
due to changes in single base pairs
(not visible by the light microscope), and not to changes in chromosomal segments as segments per se
(sometimes visible by the light microscope).
Such (C+G)% "incompatibilities at the genomic level
-- would be manifest at meiosis
when parental genomes attempt to recombine, and would affect hybrid fertility",
(i.e. sterility, not viability; Forsdyke, 1996; (Click Here). See also:
Forsdyke 1999a Abstract (Click
Here);
Forsdyke 1999b. J. Theor. Biol. 201,
47-61 (Click Here);
Forsdyke 2000. J. Theor. Biol. 204,
443-452 (below). |
|
|
|
Here, for simplicity we assume sex chromosome homomorphism (e.g.
no degeneration of the Y in the heterogametic sex).
When the speciation process begins (incipient
speciation) the homogametic sex (female) has to:
- Differentiate the sex chromosomes.
- Differentiate the
autosomes.
- Activate check-point mechanisms so that
meiosis fails.
The heterogametic sex has already
completed much of the first of these, so is en route to speciation even
before speciation initiates. The sterility means that the parents are
beginning to become reproductively isolated from each other - a major
desideratum for successful speciation. |
|
Unexpected Results
(Naveira and Maside, 1998) 
|
A
suitable experimental test of the hypothesis presented in this page, would be
progressively to change the base composition [(C+G)%] of
a line and examine the appearance of hybrid male sterility.
|
 |
Perhaps, this test has already
been carried out in the form of the "introgression"
of chromosomal segments between lines. Thus, analysis of hybrids between two fruit fly
lines (D. buzzatii and D. koepferae) produced results which were "quite unexpected".
Let us call the lines A and B, and suppose that the
result of a cross between them is the production of just females, not males. Now we want
to introduce successively larger pieces of the B genome into the A
genome, producing lines A', A'', A''',
etc. Then we cross the original line A with A' and see
if the ratio of males to females among the offspring is decreased. Then we cross A
with A'' and check the ratio again -- and so on. This will allow us to
tell how much of the B genome we have to transfer (a) to begin decreasing
the ratio, and (b) to eliminate males entirely.
The procedure known as
"introgression" allows the experiment to be
performed. This involves repeated back-crossing the hybrid offspring (female) of an A
x B cross, with males of the parental line A. The
haploid gametes of the parental line can be represented as A
and A, whereas the haploid gametes of the
hybrid offspring would be, not just A and B, but, as a result of recombination during
meiosis, A-with-varying-amounts-of-B, and B-with-varying-amounts-of-A.
The gamete type A-with-varying-amounts-of-B
united with a parental-line type A gamete
("back-cross") would result in offspring producing gametes of type A-with-even-lesser-amounts-of-B. The process is
repeated for many generations to produce the above-mentioned A, A'',
A''', etc. stock.
Next, Naveira and
Maside assessed the degree of B introgression into A, by
taking advantage of the fact that salivary gland cell chromosomes are easily visualized
(polytene chromosomes), and the degree of pairing between paternal and maternal homologs
can be seen. Where there was B introgression pairing was defective. They
noted:
"A correlation between the lengths of
individually introgressed segments and the fertility/sterility outcome was observed: long
segments produced sterility, while short segments did not -- . On average, interspecific
substitution of roughly up to 30% of any of the autosomes allows hybrid male fertility,
whereas a 40% substitution leads to hybrid male sterility.
--
The transition from
fertility to sterility as the length of the introgression is increased can be shown to be
quite sharp, thus revealing the existence of genuine threshold length intervals. What is more, the threshold can be crossed by recombining in the
genome of a hybrid male two fertile introgressions from distant locations in the same
chromosome, or even from different chromosomes." |
|
From further studies with with introgressed
non-coding DNA in other lines they further conclude that:
| "The coding potential of the
introgressions -- might be also irrelevant for hybrid male fertility. It might be only a question of foreign DNA amount
-- " |
Despite this suggestion, they refer to
their model of the "genetic basis of hybrid male sterility in Drosophila"
as "generally polygenic," to distinguish it from
the "genic" model. They are not inclined on the
basis of their evidence to refer to the model as "chromosomal".
"Current evidence suggests that the
genetic basis of hybrid male sterility in Drosophila is generally polygenic, and
the total number of sterility factors must probably be numbered at least in the hundreds.
The individual effect on fertility of any of these factors is virtually undetectable, but it can be accumulated to others. So,
hybrid male sterility results from the co-introgression of a minimum number of randomly
dispersed factors (polygenic combination). The different factors linked to the X, on the
one hand, and to the autosomes, on the other, are interchangeable
-- .
Recent experiments on the nature of these polygenes suggest that the coding
potential of their DNA may be irrelevant." |
|
Prevention
of recombination is needed to preserve both phenotypic differentiation between species and sexual phenotypic
differentiation within species. For
species differentiation (speciation), isolating barriers preventing recombination may be
prezygotic (gamete transfer barriers), or postzygotic (either a developmental barrier
resulting in hybrid inviability, or a chromosomal-pairing barrier resulting in hybrid
sterility). The sterility barrier is usually the first to appear and, although often
initially only manifest in the heterogametic sex (Haldane's rule), is finally manifest in
both sexes. For sexual differentiation, the first and only barrier is chromosomal-pairing,
and always applies to the heterogametic sex. For regions of sex chromosomes affecting
sexual differentiation there must be something analogous to the process generating the
hybrid sterility seen when allied species cross.
Explanations for Haldane's rule have generally assumed that the chromosomal-pairing
barrier initiating evolutionary divergence into species is due to incompatibilities
between gene products ("genic"), or sets of gene
products ("polygenic"), rather than between
chromosomes per se ("chromosomal"). However,
if chromosomal incompatibilities promoting incipient sexual differentiation could also
contribute to the process of incipient speciation, then a step
towards speciation would have been taken in the heterogametic sex.
Thus, incipient
speciation, manifest as hybrid sterility when "varieties"
are crossed, would appear at the earliest stage in the heterogametic sex, even in genera
with homomorphic sex chromosomes
(Haldane's rule for hybrid sterility).
In contrast, it has been proposed that Haldane's rule for hybrid
inviability needs differences in dosage compensation, so could not
apply to genera with homomorphic sex chromosomes. |
Introduction
It
has been proposed that there is a fundamental similarity between the evolutionary process
which divides two groups within a species such that they become two species, and the
evolutionary process which divides two groups within a species such that they become two
sexes (Bateson, 1904, 1908, 1922a). Here, both historical and contemporary evidence
supporting this view is marshalled. |
Sex
Chromosomes
In
many species males and females are produced in equal quantities. Mendel himself noted that
such equal quantities would be produced if a recessive homozygote were crossed with a
heterozygote (Bateson & Saunders, 1902; Bateson
1909). Thus, if red is dominant to
white, when a homozygous white (WW, producing one type of gamete, W
and W) is crossed with a heterozygous red (RW, producing two types of gamete, R and W), on
average equal numbers of red and white progeny should be produced (RW, WW). If sex were similarly
determined, this simple scheme would suggest that one sex be homozygous and the other sex
be heterozygous for alleles of a particular gene.
As long as only one allele pair were required there would be no reason to regard this
process as different from any other genetic process. The chromosome pair containing the
gene, -- let us call them X and Y chromosomes, -- would
be equal in all respects, including size ("homomorphic").
One sex would be homozygous for the recessive allele (the "homogametic"
sex) and the other sex would be heterozygous (the "heterogametic"
sex). If there were recombination between the chromosome pair in the heterogametic sex,
the gene might switch chromosomes, thus converting an X chromosome to a Y
chromosome and the corresponding Y chromosome to an X
chromosome. The status quo would be preserved and equal numbers of differentiated
gametes would still be produced.
However, if the complexities of
sexual differentiation were to require more than one gene, -- say genes X1
and X2 on the X chromosome, and the
corresponding allelic genes Y1 and Y2
on the Y chromosome, -- the situation would get more complicated.
Recombination (crossing-over between chromosomes at meiosis) might then separate the two
genes to generate chromosomes (and hence gametes) with genes X1
and Y2 together, and genes Y1
and X2 together. Sexual differentiation would be
impaired.
To prevent this happening
either the gene pairs, -- X1 and X2,
and Y1 and Y2 --
would have to be closely linked on the X and Y
chromosomes, respectively, and/or one of the chromosomes would have to develop some local
mechanism to prevent recombination (Ohno
1967). If such antirecombination activity could
not easily be localized, then the activity might spread to involve other genes on the
chromosomes; these genes might themselves play no role in sexual differentiation.
This seems to be the situation
that often prevails. The predominant function, sex determination, overrules, but does not
necessarily eliminate, the functions of other genes on the same chromosome. The
chromosomes are referred to as the sex chromosomes, even though concerned with many
functions not related to sex.
Recombination has evolved because it
is advantageous (Winge, 1917; Dougherty, 1955; Click
Here). The benefits of recombination are not to be lightly discarded. However, for the
initiation of speciation, recombination is a hazard; indeed, the prevention of
recombination between members of
different incipient species, or of an incipient species and the parental species ("reproductive isolation"), appears to be a fundamental part of
the speciation process (Forsdyke, 1996, 1998, 1999a,
b).
On the other hand, sex chromosomes
exist within a species, and
antirecombination is only beneficial to the extent that it prevents recombination between
genes specifically concerned with sexual differentiation. If antirecombination activity
should spread to encompass genes not concerned with sexual differentiation, the latter
would loose any benefits recombination might have conferred. To the extent that
recombination allows the correction of damaged genes (Bernstein & Bernstein,
1991),
any non-lethal mutations resulting from such damage would remain uncorrected. There would
then be no prevention, within species,
of further changes in sex chromosomes, including additions, deletions and other "macromutations" similar to those that occur between species as they differentiate
(Chandley et al. 1975).
Thus, instead of remaining
homomorphic, the sex chromosomes might come to differ in size (heteromorphic). In some
species (e.g. certain fish and amphibia) the sex chromosomes remain homomorphic and appear
to differ only in the genes affecting sexual differentiation. In other species (e.g.
mammals, birds) the sex chromosomes are heteromorphic. It is argued that modern
heteromorphic sex chromosomes evolved from homomorphic ancestors (Ohno
1967). |
Similarity between Species and Sexual Differentiations
Species differentiation and sexual differentiation can be confused. When
classifying organisms into species, two forms initially considered as distinct species
have sometimes been found to be merely the sexually differentiated forms of one species
(De Vries, 1910). |
 |
 |
An extreme case of this was Charles Darwin's realization (1851) that an apparent member of
a parasite species found within the body of a female barnacle was actually the male form
of that species ("complemental males").
The reason for the confusion among systematists is
that sexual and species differentiations can involve morphological and physiological
changes of similar orders. This was recognized by William Bateson (1904) at a time when
the chromosomal basis of sexual differentiation was still tentative: |
"Whenever sexual
organisms breeding together produce a mixture of forms, there is, -- prima facie reason to suspect that the mixture is due to
differentiation of germs [gametes]. The most
familiar case is sex itself. A population consisting of males and females has so many
features in common with the differentiating offspring resulting from the segregation of
characters among the germ-cells of cross-bred [hybrid]
organisms that it is impossible to avoid the suspicion that the two phenomena are similar
in causation." |
| In 1908 he further noted: |
"We may feel fairly
sure that the distinction between the sexes depends on the presence in one
or other of them of an unpaired factor -- .The results of
genetic research are so bewilderingly novel -- . In all
the discussions of the stability and fitness of species, who ever contemplated the
possibility of a wild species having one of its sexes permanently hybrid [i.e. XY or WZ chromosomes]?
-- Who would have
supposed it possible that the pollen cells of a plant could be all of one type, and it egg
cells of two types". |
This acknowledged
studies of his colleague Edith Saunders on certain dioecious plants (plants with two
independent sexes) in which the female is the heterogametic sex (i.e. has two types of
gametes), and the male is the homogametic sex (i.e. has one type of gamete; Punnett &
Bateson, 1908). Later the chromosomal basis of sexual differentiation became clearer and
in a Toronto address to the American Association for the Advancement of Science Bateson
(1922a) commented on the chromosomally-borne "ingredients"
responsible for this: |
"We have now to admit the further conception
- that between the male and female sides of the same plant these ingredients may be quite
differently apportioned, and
- that the genetical composition of each may be so distinct that the systematist might,
without extravagance, recognize them as distinct specifically [i.e. mistake the different
sexes as distinct species].
If then our plant may -- give off two distinct forms, why is
not that phenomenon [i.e. sexual differentiation]
a true instance of Darwin's origin of species?" |
As
noted above, species and sexual differentiations have an important feature in common. For
their maintenance there must be an absence of recombination, either between one or more
chromosome pairs in both sexes
(speciation), or between regions of one chromosome pair in one
sex (sexual differentiation).
-
For species differentiation, chromosomes of members of a
variant group must not recombine with those of the parental line, otherwise character
differences due to multiple genes could blend, and the differentiation would be lost.
-
For sexual differentiation, regions of the sex
chromosomes must not recombine because the characters conferring sexual identity could
blend, and sexual differentiation would then be lost. The sex chromosomes (e.g. X
and Y in humans, where the male is the heterogametic sex) must, between
themselves, maintain something akin to the reproductive isolation which defines organisms
as members of distinct species (Forsdyke,
1999b).
The most obvious features of both
differentiations are those due to differences in individual gene products, which might
include gene products affecting recombination. This has led to a "genic" view of speciation
(Coyne, 1992). An alternative "chromosomal" view postulates failure of recombination due to
lack of homology between chromosomes attempting to pair at meiosis (Darlington, 1932;
Gulick, 1932; White, 1978; King, 1993). Several objections to this view
(Coyne & Orr, 1998) have been addressed in recent work
(Forsdyke, 1996, 1998, 1999a, b). Further support
for the chromosomal view would be provided if it could explain, more economically than the
genic alternative, the phenomenon known as "Haldane's rule". |
Haldane's
Rule
If
species are defined as groups of organisms which are reproductively isolated from each
other, then the question of how species originate becomes that of how reproductive
isolation originates? This may be pictured genealogically as an inverted Y (Fig. 1) with a
vertical time-axis (Darwin, 1859). The stem of the Y indicates a single species, which at
the fork branches into two individual species, which then further differentiate into
modern forms (found at the tips of the arms). Modern horses and giraffes, for example, are
reproductively isolated because of an inability to copulate ("transfer
barrier"). However, it is unlikely that this form of reproductive isolation is
responsible for the initial branching
event in most cases of species formation. |
|
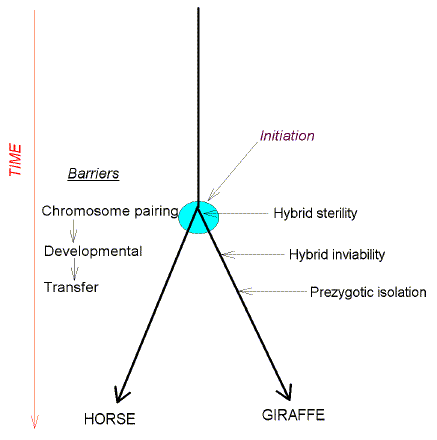
|
 |
 |
|
FIG. 1.
Hybrid sterility as the form of reproductive isolation responsible for the initiation of
speciation in most cases of species formation. For further details please see text.
|
Indeed, if we were to go back
through time (upwards, along the arms of our inverted Y genealogy), it is likely that we
would arrive at proto-horses and proto-giraffes which were able to copulate. There would
be no transfer barrier separating male gametes from female gametes, and fertilization
might then occur so that the parental genomes could come to coexist in one cell (zygote).
Thus, there would be no prezygotic reproductive isolation. However, by this time after the
initial divergence the species differentiations would have been so advanced that the
products encoded by the two genomes would not be able to cooperate to allow development to
occur. Thus, the hybrid would not survive. The transfer
barrier might not have yet arisen, but this developmental
barrier (producing "hybrid inviability") would
still have ensured reproductive isolation.
Nevertheless,
the developmental barrier is unlikely to be responsible for the
initial branching event in most
cases of species formation. If we were to go even further back towards the time of the
initial divergence, we would probably encounter proto-horses and proto-giraffes which were
able both to copulate successfully
(no transfer barrier), and to give
rise to offspring which grew to become healthy, vigorous, adults (no developmental
barrier). At this time, although the transfer and developmental barriers had not yet
arisen, there would still have been reproductive isolation. The hybrids, although
vigorous, would have been sterile ("mules"), and so
no offspring would be produced.
Thus, moving further up the arms of
our inverted Y genealogy, we encounter the barrier to reproduction most proximate to the
point of species divergence ("hybrid sterility").
We are very close to the branch point leading downwards to proto-horses and
proto-giraffes, but we are not quite at it. Hybrid sterility is not an all-or-none
phenomenon. Initially hybrid sterility is only partial, so that some fertile offspring are
produced. The first manifestation of a species branching into two lines is a degree of hybrid sterility. When examining
the offspring of a cross between such lines a remarkable regularity became apparent to a
young mathematically-gifted biologist whose mentors included Bateson (Haldane,
1957).
|

|
Although it has attracted the
attention of biologists for centuries, J. B. S. Haldane in 1922 appears to have been the
first to enunciate that "when in the F1 [first generation] offspring of two different
animal races [lines], one sex is
absent, rare or sterile, that sex is the heterozygous [heterogametic]
sex" (Craft,
1938). The absence of one sex among the
offspring can reflect a failure of the fertilized ovum (zygote) to develop properly. This
is Haldane's rule for "hybrid inviability"
(Forsdyke, 1995). We are here concerned primarily with Haldane's rule for "hybrid sterility"(Fig.
2). |
|
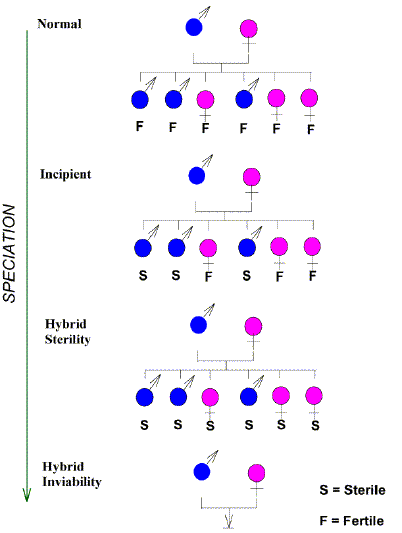
|
FIG. 2. Progression towards complete (sympatric) reproductive isolation between
two "lines", such that they become "species".
- In the normal situation (top)
there are equal numbers of males and females among the progeny of a cross, and all are
fertile (F).
- Incipient speciation is marked by preferential loss of
fertility (sterility; S) in the heterogametic sex (in this case males).
- Reproductive isolation is complete when this progresses to hybrid
sterility in all offspring (not necessarily accompanied by a decrease in number
of offspring).
- This hybrid sterility barrier may be replaced by hybrid
inviability (bottom: initially fewer viable offspring, progressing to no viable
offspring).
- In the general case (initially no allopatric isolation) these two post-zygotic
barriers might eventually be replaced by prezygotic barriers (not shown;
e.g. inability to copulate, geographic isolation).
|
The gene-centered viewpoint
has spawned a complex literature on Haldane's rule, and
|
"forms an integral part of most hypotheses, either implicitly or
explicitly" (Laurie, 1997).
|
The authors of such hypotheses have
struggled ingeniously to cope with "adaptive valleys"
and other problems intrinsic to the genic approach (Graves & O'Neill, 1997; Orr,
1997; Turelli, 1998). The lack of "wholly convincing
explanations", has led to the thought that
|
"it seems possible that some simple explanation
-- has eluded
everyone" (Coyne, 1992).
|
The much-neglected chromosomal viewpoint
can provide a simple explanation perhaps deserving consideration by those who
|
"keep an open mind about the cause(s) of Haldane's rule" (Laurie,
1997).
|
|
A Way
Station
Haldane's
rule usually concerns crosses between members of different "races"
(varieties, breeds, lines) within
what appears as a single species (defined reproductively;
Forsdyke,
1999b). We are
accustomed to recognize as "races," groups within a
species the members of which show some common morphological differentiation from the
members of other groups. In principle, members of a group ("race")
within a species might begin to differentiate with respect to reproductive potential (no
longer retaining full fertility when crossed with parental stock) before
any morphological or other physiological differences are evident (Romanes, 1886;
1897).
When fully differentiated in this way, the members of the group would be reproductively
isolated, and so would constitute a distinct species, even if not anatomically or
functionally distinguishable from members of the parental group.
Thus, Haldane's rule has no obligatory anatomical or physiological
correlates, but is a phenomenon of incipient speciation alone;
as such it is regarded as having the potential to tell us much about this process
(Coyne,
1992; Naveira & Maside, 1998). It is proposed
(Forsdyke, 1999b) that the preferential
sterility of the heterogametic sex can be regarded as a step,
or way station, on the path
towards the complete sterility associated with full species differentiation, a process
generally accompanied by the anatomical and physiological differentiation of conventional
phenotypic characters. |
Chromosomal Hypothesis for Haldane's Rule
In
the homogametic sex the two sex chromosomes are essentially identical (e.g. genotype XX
in human females), and can recombine at meiosis. The opportunity for different sex
chromosomes to recombine at meiosis arises in the heterogametic sex (genotype XY
in human males). Here, as in the homogametic sex, there would be a homology search perhaps
initiated by "kissing" interactions between the
aligned chromosomes (Forsdyke, 1996, 1998; Kleckner, 1997; Gupta, Folta-Stogniew &
Radding, 1999; Zaitsev & Kowalczykowski, 1999). If this search were successful,
double-strand breaks and recombination would occur. For the regions of the sex chromosomes
concerned with sexual differentiation to become "reproductively
isolated", it might be necessary for the homology search to fail in these
regions, either through changes in specific genes affecting the process (a "genic" cause of non-recombination; Dobzhansky, 1933; Coyne
& Orr, 1998), or through lack of homology
(a "chromosomal"
cause of non-recombination; Darlington, 1932; Gulch, 1932; White, 1978; King,
1993).
The sex chromosomes tend to become
progressively differentiated from each other, and in some species recombination remains
possible only in small "pseudoautosomal" regions
(Koller & Darlington, 1934). The non-pseudoautosomal regions of
sex chromosomes appear to be kept from pairing and recombination by a failure of homology
(a "chromosomal" hypothesis), rather
than by a failure of hypothetical gene products specifically dedicated to this pairing, but
not to the
pairing of the autosomes and pseudoautosomal regions (a "genic"
hypothesis).
While, at the initiation of sex
chromosome differentiation it is possible that there might have been early transient genic
incompatibilities which would later have been superseded by secondary "chromosomal" incompatibilities, current opinion favours the
latter as the primary cause of the
failure of the sex chromosomes within a species to recombine (Graves, Disteche &
Toder, 1998; Graves & O'Neill, 1997; Guttman & Charlesworth, 1998; Steinemann
& Steinemann, 1998, 1999).
If the mechanisms of sexual and
species differentiations were similar (and we here take the similarities to be both
chromosomal), then, since the opportunity for recombination between different sex
chromosomes (e.g. X and Y) can occur only in the sex
with both chromosomes (the heterogametic sex), by preventing
recombination that sex could be considered to have taken a step towards speciation.
Whereas, for species differentiation, the homogametic sex would have to differentiate both sex chromosomes and
autosomes, the heterogametic sex would have only the autosomes to differentiate. By virtue
of this head-start, the heterogametic
sex would be rare or absent among the progeny of crosses between an incipient species and
its parental stock (Haldane's rule).
| The
latter sentence is in error. It should read: "By virtue of this head-start,
the heterogametic sex would be sterile (Haldane's rule for hybrid
sterility)."
Apologies
DRF Aug 2003 |
On the other hand, it is possible
that the chromosomal bases of species and sexual differentiations are mechanistically
quite distinct. The process which prevents the chromosomes of members of a variant group
within a species from recombining with those of the parental line (thus providing the
"intrinsic" isolation fundamental to many forms of
incipient speciation; Romanes, 1886, 1897; Forsdyke, 1996, 1998,
1999a,b), might be quite
unrelated to the process which prevents an X chromosome recombining with
part of a Y chromosome.
In this respect it should be noted
that the differentiation of parts of the sex chromosomes within
a species clearly does not impair gametogenesis in the heterogametic sex, whereas
differentiation of sex chromosomes and autosomes in the course of speciation impairs
gametogenesis in hybrids of both sexes (hybrid sterility). Thus, the "checkpoint" leading to meiotic disruption and impaired
gametogenesis would have to be inactive in the case of sex chromosome differentiation at
least until such a time as the sex chromosome differentiation came to contribute to
species differentiation. |
Genic
versus Chromosomal
To
complete the speciation process the meiotic homology search must fail both between the
paired pseudoautosomal regions of the sex chromosomes, and between paired autosomes. The
sterility accompanying this has been recognized for thousands of years in the form of the
mule. |
 |
The cross
between a horse and an ass is productive (first crosses succeed) producing a mule.
However, the mule is sterile so that a subsequent cross would fail (second crosses fail).
The sterility is associated with a degenerate gonad (Stephan, 1902; Guyer, 1902; Chandley
et al., 1974), presumably produced by "check-point"
mechanisms which detect errors in meiosis (Page & Orr-Weaver, 1996; Smith &
Nicolas, 1998).
A case
has been made that the failure of pairing of homologous chromosomes at meiosis is of
"genic" origin (Coyne & Orr,
1998). Indeed, the
pairing of homologous chromosomes would require some gene products, and thus mutations in
these genes should impair pairing. Such genes might well be identified, mapped and
successfully cloned. However, the validity of some experiments claiming precise
localisation of genes having a major effect on hybrid sterility has been questioned
(Maside & Naveira, 1996; Naveira & Maside,
1998).
The "chromosomal
view" sees homologous chromosomes normally pairing or "conjugating" due to complementary features, likened to "the sword and the scabbard". Bateson (1922b) wondered how this
complementarity might be lost: |
"That [hybrid] sterility might quite reasonably be supposed to be
due to the inability of certain chromosomes to conjugate, and -- [the]
simile of the sword and scabbard may serve to depict the sort of thing we might expect to
happen. But the difficulty is that we have never seen it happen to swords and scabbards
which we know to have belonged originally to each other. On the contrary, they seem always
to fit each other, whatever diversities they may have acquired." |
 |
The evidence that
Bateson was seeking seemed to emerge from studies by conventional light microscopy of the
chromosomes of sterile hybrids, which revealed differences between homologous chromosomes
(e.g. Darlington, 1932; Chandley et al.,
1974). It is understandable that such obvious
chromosomal rearrangements ("macromutations"),
probably occurring after the initial
evolutionary divergence was complete, should have been proposed as candidates contributing
to the initial isolation process by advocates of the chromosomal hypothesis
(Bush et al.,
1977; White, 1978; King, 1993). However, as Coyne and Orr point out
(1998), some types of
macromutation are compatible with pairing.
The possibility of more subtle chromosome changes was suggested by studies in the fruit
fly by Naveira & Maside (1998), who concluded: |
"The total number of sterility factors [on
chromosomes] must probably be numbered at
least in the hundreds. The individual
effect on fertility of any [one] of these
factors is virtually undetectable, but can be accumulated
to others. So, hybrid male sterility results from the [experimental] co-introgression of a minimum number
of randomly dispersed factors
(polygenic combination). The different factors linked to the X, on the
other hand, and to the autosomes, on the other, are interchangeable
[i.e. are not gene-specific].
-- Recent experiments on the nature of these polygenes
suggest that the coding potential of their DNA may be
irrelevant." |
| In the latter respect, it was further
noted that: |
|
"The effect detected after inserting non-coding DNA suggests that
the coding potential of the introgressions -- might be -- irrelevant for hybrid
male fertility. It might be only a question of foreign
DNA amount, -- ."
|
In support of this
essentially chromosomal viewpoint
(although the authors refer to it as "polygenic"),
it has been shown that small differences in base composition, a known species-specific
characteristic, should suffice to disrupt pairing (Forsdyke, 1996, 1998,
1999a,b). Such
"micromutations", which change base composition
(the percentage C+G), would not be observed microscopically, but would
function collectively to disrupt pairing in the fashion suggested by Naveira and Maside
(1998).
Later-developing secondary mechanisms of isolation,
substituting for the primary micromutation-dependent mechanism, would include prezygotic
isolating factors (e.g. mating preferences constituting a transfer barrier), and
chromosomal macromutations. A strength of the micromutation version of the chromosomal
hypothesis is that it allows fine degrees of compatibility, so that a rare reproductive
variant would be more likely to find a partially matching "physiological
complement" (Romanes, 1886;
1897).
Further evidence for the chromosomal
view derives from studies in allopolyploids. Here, sterility due to genic
incompatibilities should usually still be manifest, whereas the presence of a pairing
partner should effectively "cure" sterility due to
chromosomal incompatibilities. This polyploidy test (Winge,
1917) can discriminate between
genic and chromosomal causes of hybrid sterility (Dobzhansky,
1937).
Some applications of the
polyploidy test by Dobzhansky (1933) show a genic cause to apply in his particular cases
of sterility. Other applications show that sterility may also have a chromosomal basis
(Darlington, 1932; Dobzhansky, 1937). These results are consistent with cytological
studies of gametogenesis in hybrid mice (Hale et al., 1993; Matsuda et al., 1991,
1992). |
General
Model
From
the chromosomal viewpoint it follows that for speciation the homogametic
sex has to complete three steps,
- 1) differentiation of the sex chromosomes,
- 2) differentiation of autosomal pairs, and
- 3) activation of "check-points"
(Page & Orr-Weaver, 1996) which respond to such differentiations by disrupting
meiosis.
In contrast the heterogametic
sex, being already advanced in the first step, has essentially to complete
only the latter two steps. Thus, incipient speciation, manifest as hybrid sterility,
should be most apparent in the heterogametic sex.
Many observations in the Haldane's
rule literature can be reassessed from this perspective. For example, recent studies of
Presgraves and Orr (1998) showed that mosquito genera with homomorphic sex chromosomes
display hybrid sterility, but not
hybrid inviability. This is
consistent with the above hypothesis, and is also consistent with hybrid inviability being
based on differences in dosage compensation (which probably requires heteromorphic sex
chromosomes; Forsdyke, 1995). The proposed chemical basis for the primary chromosomal
change (differences in (C+G)%; Forsdyke, 1996,
1998), lends itself well
to the present model. |
Further
Questions
Although
some members of the genic school believe that "the main causes
of Haldane's rule now seem clear", it is admitted that "many ancillary questions await further analysis"
(Turelli, 1998). Presented as an economical explanation for Haldane's rule, the arguments set out
above for the chromosomal hypothesis also provoke further questions.
When the speciation process approaches
completion, the pairing of all
chromosomes is impaired at meiosis in hybrids of both sexes. Yet, if the requirements of
sexual differentiation can be satisfied by one "way station",
namely development of incompatibility in only one chromosome pair (the sex chromosomes),
why should the initial requirements of species differentiation not be satisfied by another
"way station", namely development of
incompatibility in only one autosome
pair? This should suffice to activate check-point mechanisms and disrupt gametogenesis.
Must the initiation of speciation
necessarily be accompanied by differentiation of all
the autosomes?
The results of Naveira and Maside
(1998) suggest not. A second "way station"
involving only one autosome pair should indeed suffice to complete the speciation process.
Under the shelter of the reproductive isolation so afforded, the remaining chromosomes
should rapidly develop disparities (both micromutations and macromutations). It seems
unlikely that the meiotic checkpoint(s) would require activation by signals from several
chromosomes for gametogenesis to be disrupted.
It should be noted that the
homology search mechanism, perhaps needing coordinated stem-loop extrusions under negative
supercoiling (a long-range effect of locally-acting topoisomerases or transcription
complexes; Forsdyke, 1996, 1998; Strick et al., 1998; Wong et al., 1998), implicates large
chromosomal regions. In this way the initially-developing anti-recombination activity in
homomorphic sex chromosomes might have spread and so facililitated the transition to
heteromorphism.
I thank Harcourt-Brace Inc. for granting copyright
permission to place full-text versions of relevant preceding papers at my web-site. |
REFERENCES
BATESON, W. (1904). Draft application to the Balfour Fund. In: William Bateson, F. R. S. Naturalist. His Essays and Addresses
(Bateson, B., ed.), p. 94. Cambridge: Cambridge University Press, 1928.
BATESON, W. (1908). The methods and scope of genetics. Inaugural lecture at the
University of Cambridge. In: William Bateson, F. R. S.
Naturalist. His Essays and Addresses (Bateson, B., ed.), pp. 317-333.
Cambridge: Cambridge University Press, 1928.
BATESON, W. (1909). Mendel's Principles of
Heredity, pp. 164-195. Cambridge: Cambridge University Press.
BATESON, W. (1922a). Evolutionary faith and modern doubts. Nature
109, 553-556.
BATESON, W. (1922b) Interspecific sterility. Nature
110, 76.
BATESON, W. & SAUNDERS, E. R. (1902) Report I. In: Reports
to the Evolution Committee of the Royal Society, pp. 1-160. London:
Harrison.
BERNSTEIN, C. & BERNSTEIN, H. (1991). Aging,
Sex and DNA Repair. San Diego: Academic Press.
BUSH, G. L., CASE, S. M., WILSON, A. C. & PATTON, J. L. (1977). Rapid
speciation and chromosomal evolution in mammals. Proc.
Natl. Acad. Sci. USA, 74, 3942-3946.
CHANDLEY, A. C., JONES, R. C., DOTT, H. M., ALLEN, W. R. & SHORT, R. V.
(1974). Meiosis in interspecific equine hybrids. 1. The male mule (Equus asinus X E.
caballus) and hinny (E. caballus X E. asinus). Cytogen.
Cell Genet. 13, 330-341.
COYNE, J. A. (1992). Genetics and speciation. Nature
355, 511-515.
COYNE, J. A. & ORR, H. A., (1998). The evolutionary genetics of speciation.
Phil. Trans. R. Soc. B (London) 353,
287-305.
CRAFT, W. A. (1938). The sex ratio in mules and other hybrid mammals. Q. Rev. Biol. 13,19-39.
DARLINGTON, C. D. (1932). Recent Advances in
Cytology, pp. 448-485. London: Churchill.
DARWIN, C. (1851). A Monograph on the Subclass
Cirripedia, vol. 1, pp. 281-293. London: The Ray Society.
DARWIN, C. (1859). The Origin of Species by
Means of Natural Selection. London: Murray.
DE VRIES, H. (1910). Intracellular Pangenesis
(Gager, C. S., translator), pp. 18-19. Chicago: Open Court.
DOBZHANSKY, T. (1933). On the sterility of the interracial hybrids in Drosophila
pseudoobscura. Proc. Natl. Acad. Sci. USA 19,
397-403.
DOBZHANSKY, T. (1937). Genetics and the Origin
of Species, pp. 288-291. New York: Columbia University Press.
DOUGHERTY, E. C. (1955). Comparative evolution and the origin of sexuality. Syst. Zool. 4, 145-190.
FORSDYKE, D. R. (1995). Fine tuning of intracellular protein concentrations, a
collective protein function involved in aneuploid lethality, sex-determination and
speciation? J. theor. Biol. 172,
335-345.
FORSDYKE, D. R. (1996). Different biological species "broadcast"
their DNAs at different (G+C)% "wavelengths".
J. theor. Biol. 178, 405-417.
FORSDYKE, D. R. (1998). An alternative way of thinking about stem-loops in DNA:
a case study of the G0S2 gene. J. theor. Biol.
192, 489-504.
FORSDYKE, D. R. (1999a). "The Origin of Species", revisited. Queen's Quarterly 106, 112-133.
FORSDYKE, D. R. (1999b). Two levels of information in DNA: relationship of
Romanes' "instrinsic" variability of the
reproductive system, and Bateson's "residue"
to the species-dependent component of the base composition, (C+G)%. J. theor. Biol. 201, 47-61.
GRAVES, J. A. M., DISTECHE, C. M., & TODER, R. (1998). Gene dosage in the
evolution and function of mammalian sex chromosomes. Cytogenet.
Cell Genet. 80, 94-103.
GRAVES, J. A. M. & O'NEILL, R. J. W. (1997). Sex chromosome evolution and
Haldane's rule. J. Hered. 88, 356-360.
GULICK, A. (1932). Evolutionist and Missionary,
John Thomas Gulick, pp. 498. Chicago: University of Chicago Press.
GUPTA, R. C., FOLTA-STOGNIEW, E. & RADDING, C. M. (1999). Human Rad51
protein can form homologous joints in the absence of net stand exchange. J. Biol. Chem. 274, 1248-1256.
GUTTMAN, D. S. & CHARLESWORTH, D. (1998). A X-linked gene
with a degenerate Y-linked homologue in a dioecious plant. Nature 393, 263-266.
GUYER, M. F. (1902). Hybridism and the germ cell. Bull.
Univ. Cincinnati 21, 1-20.
HALDANE, J. B. S. (1922). Sex ratio and unisexual sterility in hybrid animals, J. Genet. 12, 101-109.
HALDANE, J. B. S. (1957). The theory of evolution, before and after Bateson. J. Genet. 56, 11-27.
HALE, D. W., WASHBURN, L. L. & EICHER, E. M. (1993). Meiotic abnormalities
in hybrid mice of the C57BL/6J x Mus spretus cross suggest a cytogenetic basis for
Haldane's rule of hybrid sterility. Cytogen. Cell
Genet. 63, 221-234.
KING, M. (1993). Species Evolution. The Role of
Chromosome Change. Cambridge: Cambridge University Press.
KLECKNER, N. (1997). Interactions between and along chromosomes during meiosis. Harvey Lect. 91, 21-45.
KOLLER, P. C. & DARLINGTON, C. D. (1934). The genetical and mechanical
properties of the sex chromosomes. 1. Rattus norvegicus. J.
Genet. 29, 159-173.
LAURIE, C. C. (1997). The weaker sex is heterogametic. 75 years of Haldane's
rule. Genetics 147, 937-951.
MASIDE, X. R. & NAVEIRA, H. F. (1996). A polygenic basis of hybrid sterility
may give rise to spurious localizations of sterility factors. Heredity
77, 488-492.
MATSUDA, Y., HIROBE, T. & CHAPMAN, V. M. (1991). Genetic basis of X-Y
chromosome dissociation and male sterility in interspecific hybrids. Proc.
Natl. Acad. Sci. USA 88, 4890-4854.
MATSUDA, Y., MOENS, P. B. & CHAPMAN, V. M. (1992). Deficiency of X
and chromosome pairing at meiotic prophase in spermatocytes of sterile interspecific
hybrids between laboratory mice (Mus domesticus) and Mus spretus. Chromosoma 101, 483-492.
NAVEIRA, H. F. & MASIDE, X. R. (1998). The genetics of hybrid male sterility
in Drosophila. In: Endless Forms: Species and
Speciation. (Howard, D. J. & Berlocher, S. H., eds.), pp. 330-338.
Oxford: Oxford University Press.
OHNO, S. (1967). Sex Chromosomes and Sex-Linked
Genes, pp. 1-23. New York: Springer-Verlag.
ORR, H. A. (1997). Haldane's rule. Annu. Rev.
Ecol. Syst. 28, 195-215.
PAGE, A. W. & ORR-WEAVER, T. L. (1996). Stopping and starting the meiotic
cycle. Curr. Opin.Genet. Devel. 7,
23-31.
PRESGRAVES, D. C. & ORR, H. A., Haldane's rule in taxa lacking a hemizygous X.
Science 282, 952-954.
PUNNETT, R. C. & BATESON, W. (1908). The heredity of sex. Science 27, 785-787.
ROMANES, G. J. (1886). Physiological selection. An additional suggestion on the
origin of species. J. Linn. Soc. Zool.
19, 337-411.
ROMANES, G. J. (1897). Darwin, and After
Darwin. Vol. 3. Post-Darwinian Questions. Isolation
and Physiological Selection. London: Longmans, Green.
SMITH, K. N. & NICOLAS, A. (1998). Recombination at work for meiosis. Curr. Opin. Genet. Devel. 8, 200-211.
STEINEMANN, M. & STEINEMANN, S. (1998). Enigma of Y chromosome degeneration:
Neo-Y and neo-X chromosomes of Drosophila miranda
a model for sex chromosome evolution. Genetica 102,
409-420.
STEINEMANN, S. & STEINEMANN, M. (1999). The Amylase gene cluster on
the evolving sex chromosomes of Drosophila miranda. Genetics
151, 151-161.
STEPHAN, P. (1902). Sur la structure histologique du testicule des mulets. Compt. Rendu. Ass. Anat. 4, 37-46.
STRICK, T. R., CROQUETTE, V. & BENSIMON, D. (1998). Homologous pairing in
stretched supercoiled DNA. Proc. Natl. Acad. Sci. USA.
95, 10579-10583.
TURELLI, M. (1998). The causes of Haldane's rule, Science
282, 889-891.
WHITE, M. J. D. (1978). Modes of Speciation, pp. 323-349. San Francisco:
Freeman.
WINGE, O. (1917). The chromosomes, their number and general importance. Comp. Rend. Trav. Carls. Lab. 13,131-275.
WONG, B. C., CHIU, S-K. & CHOW, S. A. (1998). The role of negative
superhelicity and length of homology in the formation of paranemic joints promoted by RecA
protein. J. Biol. Chem. 273,
12020-12127.
ZAITSEV, E. N. & KOWALCZYKOWSKI, S. C. (1999). The simultaneous binding of
two double-stranded DNA molecules by Escherichia coli RecA protein. J. Mol. Biol. 287, 21-31.
|

|
| Enunciating Haldane's Rule in Trafalgar Square |
|
End
Note (2005)
Independent evidence for the above argument appeared in a paper by
Ironside &
Filatov (Genetics
171,
705-713). Sex chromosomes of certain species of the plant genus
Silene are 'homomorphic' to the extent that X and Y
share similar genes (i.e. genes are not hemizygous in the
heterogametic sex), although there may be minor size differences between
these chromosomes (see below). The authors found high divergence
in non-coding regions of X and Y homologues, with differentiation
between species being less for the X than for the Y homologues. It
was
concluded:
|
"The
higher interspecific divergence of DD44Y, compared to DD44X,
supports the hypothesis that Y chromosome differentiation
between incipient species precedes
reproductive isolation of the entire genome, forming an early
stage in the process of speciation." |
|
| End
Note (Dec 2010)
Plants in the genus Silene with sex
chromosomes follow Haldane's rule, so the rule applies both to animals and
plants (Brothers & Delph,
Evolution
64,
3643-48). |
|
End Note (2016)
Further evidence for the above argument appeared in a paper by Delph &
Demuth (Journal
of Heredity
107,
383-391) under the heading "Faster Heterogametic
Sex Theory." It is
concluded:
|
"There
are 3 ways that simply being heterogametic may foster the
evolution of Haldane's Rule. The first, originally favored by
Haldane (1922), is that non-coevolved X and Y (or Z and W) may
have difficulty with proper segregation during meiosis. Haldane
later suggested that this explanation was "inadequate" and
evidence from Drosophila mutants with Y to X chromosome
translocations caused him to favor a second, more generic,
suggestion that the rule is a consequence of "interspecific
differences in the sex chromosomes" Haldane (1932). Since
translocations from the Y to the X (or W to Z) in 1 species will
result in heterogametic hybrids that are missing part of their
genome, the heterogametic sex potentially evolves postzygotic
isolation faster than the homogametic sex. Although the original
chromosomal mechanism per se (i.e. mispairing of XY or ZW in
hybrids) largely fell out of favor, recent work has argued for
its importance on theoretical grounds (Forsdyke 2000) and it is
the best explanation for male rarity in some Silene hybrids
noted above (Demuth et al. 2014)." |
Hu and Filatov (2016) found
that a "large-X effect in plants" had "increased species divergence and reduced gene flow on the
Silene X-chromosome," but gene flow involving autosomal
loci was "sufficient to homogenize the gene pools of the two
species." (Molecular Ecology
25,
2609-19). Armstrong and Filotov (2008) had noted the Y
chromosome to be 38% larger than the X chromosome and
considered:
|
"Thus, currently there is little evidence for active genetic
degeneration of S. latifolia Y chromosome. Accumulation
of repetitive 'junk' DNA on the S. latifolia Y
chromosome reported by a number of studies (Hobza et al., 2006;
Kejnovsky et al., 2006) cannot be taken as evidence for
degeneration, as accumulation of 'junk' DNA on the Y does not
automatically lead to gene loss or dysfunctionalisation.
|
The sex chromosomes were implicated in the initiation of
divergence into species. The possibility that, irrespective of
genic effects, accumulation of 'junk' DNA could impair mieotic
pairing between X and Y chromosomes was not excluded. Whether
this accumulation had been favoured, even earlier, by non-genic or
genic differences in GC% (that might have impaired pairing) was
not considered.
|
|
End Note (Feb. 2017)
The "only a question of foreign DNA amount" argument of Naviera
and Masade (1998) is supported by the independent fruitfly studies of
Amanda Moehring (2011) who writes of "different amounts of
heterospecific genome" and the mouse studies of Forejt and coworkers,
who write (2013):
|
"The
asynapsis may represent a universal mechanistic basis of F1
hybrid sterility manifested by pachytene arrest. It is tempting
to speculate that a fast-evolving subset of the noncoding
genomic sequence important for chromosome pairing and synapsis
may be the culprit." |
Moehring AJ (2011) Heterozygosity and its unexpected
correlations with hybrid sterility. Evolution 65: 2621-2630.
Bhattacharyya et al. (2013) Mechanistic basis of infertility of mouse
intersubspecific hybrids. Proc Nat Acad Sci USA 110:E468-E477
|
Who is the
Lady?
The lady is Louisa K. Haldane,
mother of J. B. S. Haldane, and friend of George John Romanes (Click Here). JBS grew
up with the latter's youngest sons at Oxford (Norman Hugh and Edmund Giles), and went to Eton with them
(Click Here).
Although he never knew Romanes himself (he was two when Romanes died),
JBS knew William Bateson very well. Remarkably, JBS seems to have been the last great
disparager of Romanes' evolutionary views. For more on this see my
article on Haldane in the Nature
Encyclopedia of Life Sciences
(2001; Click Here), or the
Nature Encyclopedia of the Human Genome (2002).
Donald Forsdyke |

|
Return to: X-Chromosome
Index (Click Here)
Return to : Evolution
Index (Click Here)
Return to: Homepage
(Click Here)
This page was established circa
1999 and last edited on
10 Nov 2020 by Donald Forsdyke.
WWW page access counter Since 11th May 1999
Since 11th May 1999