DISCUSSION
That immune responses in vivo can be critically
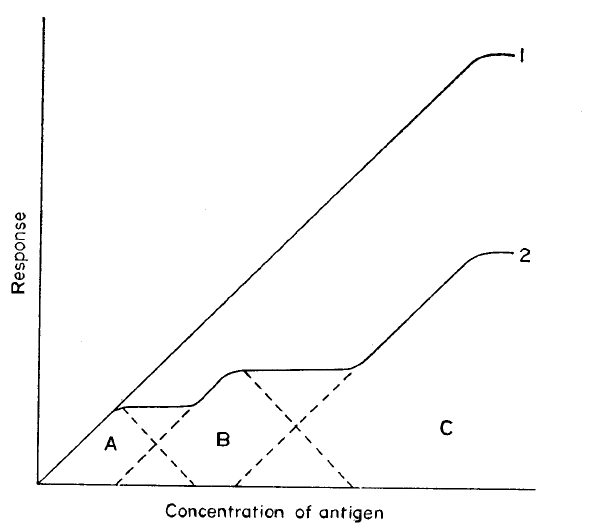
dependent on antigen concentration is well documented (14,15). There are a
number of in-vitro studies which may be interpreted as showing that lymphoid cells respond
to low concentrations of antigen and are inhibited at higher concentrations:
- It has been shown that whereas antigen added directly to a suspension of lymphoid cells
containing macrophages will not induce a primary immune response, the antigen extracted in
lower concentrations from antigen-treated peritoneal-exudate cells will induce such a
response. When the concentration of antigen added to the peritoneal cells was increased,
the ability of the extract to induce a response disappeared (16-20).
- Phenol extracts of the spleens of antigen-treated animals also appeared inhibitory in an
in-vitro lymphoid-cell system. On dilution, the extracts could induce a primary immune
response (21).
- Others have found that a primary response of lymphoid-tissue fragments to erythrocytes
is only demonstrable by lowering the ratio of antigen concentration to tissue
concentration (22).
- Experiments designed to examine more precisely the antigen dose-response requirements of
in-vitro immune responses have provided less controversial evidence. The secondary
response in vitro of circulating blood leucocytes showed an inhibition at high antigen
concentrations (23,
24); but attempts to demonstrate the specificity of
this inhibition were unsuccessful (25). Recently, however, the specificity
of inhibition by excess antigen has been shown in an in-vitro primary-response system
(26).
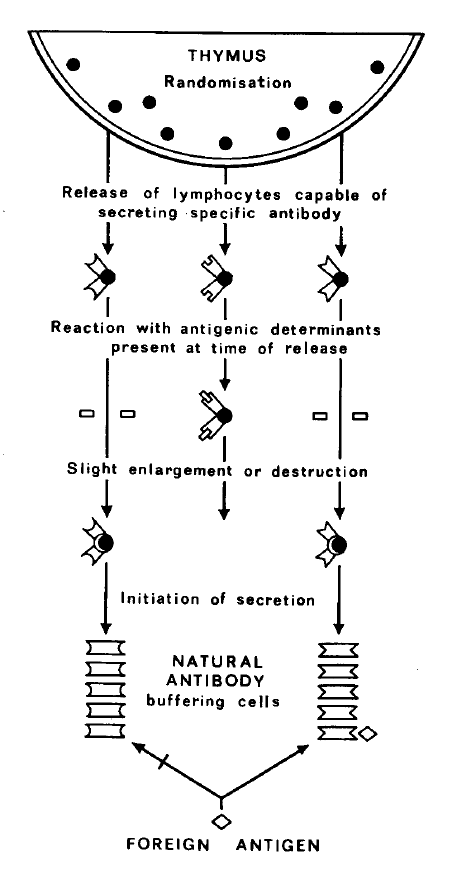
Although a case can be made out for the existence of natural
antibody (27, 28), there is no evidence that it buffers cells. However, an
interesting analogy with the postulated cell buffering has arisen from studies of the
in-vitro activation of lymphocytes by phytohaemagglutinin. The transforming activity of
solutions of phytohaemagglutinin could be adsorbed out with leucocytes (29, 30);
this indicated that the activating principle might produce its effect by reacting with
cells directly. However, the actual quantity of phytohaemagglutinin required to produce a
given activation response of cells was not proportional to the cell concentration, but to
the concentration of serum in the culture medium. The response was proportional to the
phytohaemagglutinin/serum concentration ratio (31-33).
The requirement of the combination of two antigenic determinants with
two cell-borne antibody sites for cell destruction, is compatible with current knowledge
of the action of complement on cells. It is likely that not one, but two, separate
reactions in close proximity of antigenic determinants with antibody sites must occur for
complement to be fixed and destroy a cell (34,
35). In the presence of
complement, anti-lymphocyte antibody stimulates lymphocytes to transform in vitro;
however, at high antibody concentrations cells are destroyed (36-38).
Anti-immunoglobulin antibody also activates lymphocytes to transform in vitro, and
evidence has been obtained suggesting a reaction of the anti-immunoglobulin antibody with
a cell-borne immunoglobulin (39).
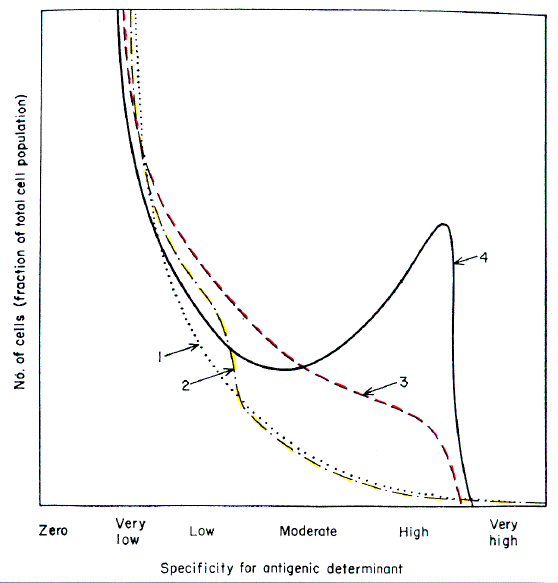
Fig. 2 summarizes the main outlines of the hypothesis. A preliminary
account of this work has been published (32). A more extensive hypothesis
is presented elsewhere (40).
References
1. Jerne, N. K. Proc. natn. Acad. Sci., Wash. 1955, 41,
849.
2. Burnet, F. M, The Clonal Selection Theory of Acquired
Immunity. 1959.
3. Lederberg, J. Science, N. Y. 1959, 129, 1649.
4. Eisen, H. N., Karush, F. Nature, Lond. 1964, 202, 677.
5. Claman, H. N., Talmage, D. W. Science, N.Y. 1963, 141, 1193.
6. Cross, A. M., Leuchars, E., Miller, J. F. A. P. J. exp. Med. 1964, 119,
837.
7. Nossal, J. G. V., Szenberg, A., Ada, G. L. G., Austin, C. M. ibid. 1963, 119,
485.
8. Friedman, H. P. Experientia, Basel, 1964, 20, 564.
9. Nossal, J. G. V. Ann. N.Y. Acad. Sci. 1964, 120, 171.
10. Murray, R. G., Woods, P. A. Anat. Rec. 1964, 150, 113.
11. Bell, C. G., Hayes, F. N. (editors). Liquid
Scintillation Counting. London, 1958.
12. Morton, G. A., Robinson, K. W. Nucleonics, 1949, 4, 25.
13. Hales, C. N., Randle, P. J. Lancet, 1963, i, 790.
14. Dixon, F. J., Maurer, P. H. J. exp. Med. 1955, 101, 245.
15. Mitchison, N. A. Proc. R. Soc. B. 1964, 161, 275.
16. Fishman, M. J. exp. Med. 1961, 114, 837.
17. Fishman, M., Adler, F. L. ibid. 1963, 117, 595.
18. Fishman, M., Van Rood, J. J., Adler, F. L. in Molecular
and Cellular Basis of Antibody Formation (edited by J. Sterzl); p.
491. New York, 1965.
19. Askonas, B. A., Rhodes, J. M. Nature, Lond. 1965, 205, 470.
20. Friedman, H. P., Stavitsky, A. B., Solomon, J. M. Science, N. Y. 1965, 149,
1106.
21. Friedman, H. P. ibid. 1964, 146, 934.
22. Globerson, A., Auerbach, R. J. exp. Med. 1966, 124, 1001.
23. Cowling, D., Quaglino, D. J. Path. Bact. 1965, 89, 63.
24. Caron, G. A. Int. Archs Allerg. 1966, 30, 331.
25. Caron, G. A. ibid. 1967, 31, 441.
26. Diener, E., Armstrong W. D. Lancet, 1967, ii, 1281.
27. Boyden, S. V. Adv. Immunol. 1966, 5, 1.
28. Kerman, R., Segre, D., Myers, W. L. Science, N.Y. 1967, 156, 1514.
29. Kolodny, R. S., Hirschhorn, K. Nature, Lond. 1964, 201, 715.
30. Nordman, C. T., De La Chapelle, A., Grasbeck, R. Acta med. scand. 1964,
supplement no. 412, p. 49.
31. Forsdyke, D. R. Lancet, 1966, i, 715.
32. Forsdyke, D. R. in The Biological Effects of
Phytohaemagglutinin (edited by M. W. Elves); pp. 115, 195.
R. Jones and A. Hunt Orthopaedic Hospital, Oswestry, 1966.
33. Forsdyke, D. R. Biochem. J. 1967, 105, 679.
34. Humphrey, J. H., Dourmashkin, R. R. in Ciba Fdn Symp.
Complement (edited by G. E. W. Wolstenholme and J.
Knight); p. 175. London, 1965.
35. Borsos, T., Rapp, H. J. Science, N. Y. 1966, 150, 505.
36. Grasbeck, R., Nordman, C., De La Chapelle, A. Acta med. Scand. 1964, supplement
no. 412, p. 39.
37. Holt, L. J., Ling, N. R., Stanworth, D. R. Immunochemistry, 1966, 3,
359.
38. Forsdyke, D. R. Unpublished, 1967.
39. Sell, S., Gell, P. G. H. J. exp. Med. 1965, 122, 923.
40. Forsdyke, D. R. Ph. D. thesis,
Cambridge, 1967. Click Here [This provided the basis for a paper in the J.Theoretical
Biology in 1969.] |