
Paleolimnology
Paleolimnology is the analysis of biological, chemical, and physical indicators preserved in the sediment archive of a lake. Sediment is continually deposited at the bottom of the lake through time, and integrates materials from within the lake (autochtonous materials) and from outside the lake (allochtonous materials). By comparing changes in sedimentary materials through time, we can gain a better understanding of how the whole aquatic ecosystem is responding to various stressors.
Field work
At each study lake, we collect a variety of limnological data: measures of pH, Secchi depth, conductivity, and water temperature. We also measure dissolved oxygen concentrations and temperature at 1 m intervals throughout the water column with a YSI probe. Finally, we collect water samples to test them for a full suite of water chemistry measures (including nutrients, major ions, metals). Each lake is also cored at the deepest point in the lake, where the maximum sedimentary material collects. Sediment cores are then sectioned into 0.5 cm intervals for further analyses.



Radioisotope dating
In order to track changes in sedimentary materials through time, we must first be able to reliably assign dates to sectioned intervals. Radioisotopes such as 210-Pb are deposited from the atmosphere into lake sediments, and decay at a known rate. By measuring the activity of different isotopes, we can calculate the approximate date each sediment interval was deposited. This allows us to pinpoint when changes in our indicators are occurring.
Fossil pigments (chlorophyll-a)
Chlorophyll-a is a photosynthetic pigment that is mostly ubiquitous in all plants and algae. It is therefore considered a reliable proxy for whole-lake primary production (Michelutti and Smol. 2016). To track changes in chlorophyll-a in the sedimentary record, we use visible near-infrared spectroscopy, as chlorophyll-a concentrations can be inferred from characteristic troughs at 650-700 nm in the absorption spectra of freeze-dried sediment samples (Michelutti et al. 2010).
Biological subfossils
Paleolimnologists use a variety of biological indicators to track environmental change in the sediment record. There are a number of characteristics that suitable indicator taxa possess, including:
- Preserve well and in abundance in the sediment record.
- Can be identified to an ecologically significant level
- Sensitive to environmental variables & have diverse tolerance ranges
- Respond rapidly due to short generation times
- Numbers in sediment subsamples can be considered representative of whole-system relative abundances
The biological indicators used in the present study include:
Diatoms
Diatoms are single-celled primary producers that produce siliceous (silica based) cell walls that preserve well in sediment for millennia. They are the most common paleolimnological biological indicator because they are present in almost all aquatic environments, and are sensitive to environmental change at short time scales. Diatoms are well-established as indicators for climate change, acidification, and eutrophication (Battarbee et al. 2001).
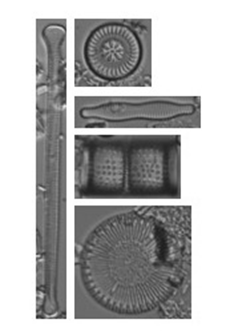
Chrysophytes
Scaled chrysophytes also produce siliceous subfossils, and are useful indicators of climate change and eutrophication (Zeeb and Smol 2001). When present in a core, siliceous scales can be identified to species level. Stomatocysts, or resting stages, are also siliceous but cannot be reliably identified to species-level. Instead, stomatocysts are commonly quantified as a diatom:cyst ratio, These chrysophyte indices can be indicative of water temperature, nutrient status, pH, and more.
Midges: Chironomidae and Chaoboridae
Chitinous remains of Chironomid (non-biting midges) and Chaoborid (phantom midges) aquatic larvae are abundant in lake sediments and easily identifiable to the subfamily, genus or species-level (Brodersen and Quinlan, 2006). Head capsules of chironomids and mandibles of chaoborids are especially useful indicators of the hypolimnetic dissolved oxygen declines associated with eutrophication. These assemblages can also be used to track climate change, salinity, and acidification.

Cladocerans: Chydoridae, Bosminidae
Cladocera (water fleas) are aquatic crustaceans. Their carapaces and head shields preserve well in the sediment record, and while the carapaces are more abundant, the head shields are more reliably identified to species-level.
Sterols and stanols
Sterols and stanols are organic molecules that occur at different characteristic ratios in different biological groups. The molecules are subcompounds of steroids, with the most widely recognized animal sterol identified as cholesterol, as well as its plant equivalent. These compounds remain stable over time and can therefore be used as biomarkers indicative of the source of organic matter in sediment. This approach has been previously used to track seabird populations in the high Arctic (Cheng et al. 2016). In this project, we will develop geochemical profiles, or environment forensic fingerprints, of different sterol and stanol compounds in our study lakes linked to stable isotope analysis. This will indicate if organic matter is sourced from a) mainly algae and/or terrestrial plants, b) livestock associated with conventional agriculture, or c) mink farming waste (e.g. carcasses, feces).
Contaminants: Metals and persistent organic pollutants (POPs)
Persistent organic pollutants (POPs) are anthropogenic contaminants that are highly lipophilic, meaning they adhere to biological tissues and remain in ecosystems for long periods of time. Metals (e.g. arsenic, cadmium, mercury) also have potential to cause substantial harm in aquatic ecosystems. When these types of contaminants are introduced to lower trophic levels (e.g., algae), they have the potential to move up the food chain and bioaccumulate in higher trophic levels (e.g., fish or birds). Captive mink are fed marine fishmeal diets that may contain high concentrations of contaminants, which may be introduced to lakes via waste effluents. Using paleolimnological techniques, we will reconstruct profiles of metal and POP concentrations in sediments.
Stable isotopes
The marine fishmeal diet of captive mink can also be traced using isotopes such as δ13C and δ15N. For example, the proportion of 14N to 15N (i.e., δ15N) in sedimentary organic matter proxies changes in the trophic state of the system. This indicates whether inputs are mink-related or from other anthropogenic or non-anthropogenic sources.
Gull Biotransport
A variety of methods will be used to investigate the interaction of gulls, mink farms and biotransport. Three main areas will be assessed related to biotransport: 1) movement and behaviour 2) diet 3) material movement
Movement
To understand the movement of Herring (Larus argentatus) and Great black-backed gulls (L. marinus) we will trap gulls in known breeding colonies including Bear Island and Brier Islands and attach GPS and VHF tags. This data will be used to assess if gulls travel to mink farms. Preliminary results from data collected from four birds with solar powered Ecotone tags (below) have shown that gulls from the Bear Island colony do travel to nearby mink farms.


Activity at mink farms
Focal and scan sampling methods will be used to determine how gulls use mink farms. Focal sampling is when researchers focus on one bird for a set amount of time and record what that bird is doing and more specifically, what the bird is foraging on. Scan sampling will be used to determine how much time birds are spending on each activity and includes scanning an entire flock and recording activity of each bird.
Diet
Stable isotopes and prey collection will be used to determine gull diet. Small blood samples will be safely taken from gulls' brachial vein at the time of tag deployment and analyzed with prey samples. Stable isotopes techniques are useful to determine diet of animals and the contributions from different prey items. They also give into insight marine vs. terrestrial and trophic level feeding habitats in the weeks prior to sample collection.
Movement of materials
Soil samples will be taken from colonies and nearby reference sites to compare the materials deposited by gulls through (Figure X). Samples will be analyzed for a range of POPs and trace elements along with fecal samples from birds.

Watershed Modeling
The modeling component of the project will involve the construction and validation of a computer model that can be used to predict how watershed restoration strategies may improve water quality. The modeling component will focus specifically on phosphorus as it has been documented that concentrations of total phosphorus in some lakes in the region are elevated, and the key role that phosphorus plays in accelerated eutrophication. Lakeshore Capacity Models have been applied in the province for land use planning in urban centers, such as Halifax. The current version of the model applied by the Centre for Water Resources Studies is the product of several refinements to the modeling approach developed specifically for Nova Scotia.
Model Development
The application of the model within rural watersheds characterized by agricultural and rural residential sources of phosphorus has been a challenge due to uncertainty in selecting export coefficients from these land uses. In this project we will focus on validation of a new sub-model that predicts phosphorus loading from on-site wastewater systems based on the age of the system, wastewater loading rate, type of treatment system, and landscape attributes (topography, soils, distance to watercourse). We will also attempt to develop phosphorus loading estimates from intensive livestock operations, such as mink farms. Watershed and sub-watershed boundaries will be delineated, and soils, geology and residential development characterized, using publicly available digital elevation models and spatial data sets. The temporal progression of development will also be assessed in each watershed using aerial photography in combination with satellite imagery for more recent time periods.
Model Validation and Development of Watershed Management Strategies
One approach that could be used to develop/validate phosphorus export coefficients for these sources is to calibrate the model against historical in-lake phosphorus concentrations measured at various points in time, representing different levels of watershed development (residential, mink farming). Unfortunately, this approach has typically been limited by a lack of historical water quality data. To address this data gap, we will use the information from the paleolimnological work to derive these model parameters. Diatom-inferred total phosphorus concentrations will be the primary paleolimnological output used to calibrate the model, with additional paleo tools (δ15N ratios, stanols and sterols) providing supporting evidence of dominant pollution sources. Once validated, the model will then be used to determine the levels of phosphorus reductions that are required to achieve pre-development trophic states in both headwater and downstream lakes in watersheds impacted by mink ranching.
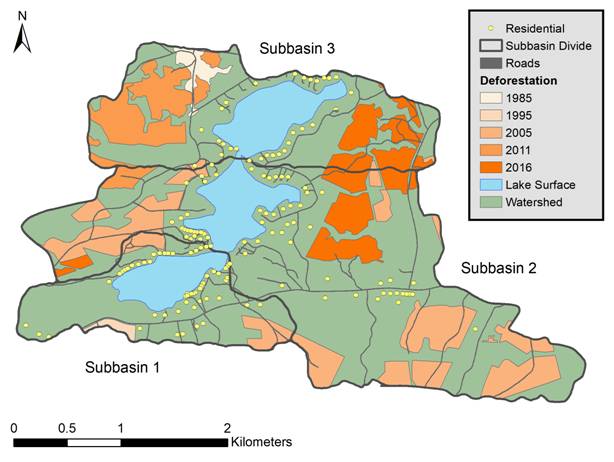
Spatial analysis of land-use change and phosphorus sources in a developing watershed
Literature Cited
Battarbee, R., Jones, V., Flower, R., et al. 2001. Diatoms. In: Tracking Environmental Change in Lake Sediments. Kluwer Academic Publishers, Dordrecht
Brodersen KP, Quinlan R. 2006. Midges as palaeoindicators of lake productivity, eutrophication and hypolimnetic oxygen. Quaternary Science Reviews 25.
Cheng, W., L. Sun, L. E. Kimpe, M. L. Mallory, J. P. Smol, L. R. Gallant, J. Li, and J. M. Blais. 2016. Sterols and stanols preserved in pond sediments track seabird biovectors in a high arctic environment. Environmental Science and Technology 50:9351–9360.
Michelutti, N., Blais, J.M., Cumming, B.F., Paterson, A.M., Rühland, K., Wolfe, A.P., and Smol, J.P. 2010. Do spectrally-inferred determinations of chlorophyll a reflect trends in lake trophic status? J. Paleolimnology 43: 205-217.
Michelutti, N., and J. P. Smol. 2016. Visible spectroscopy reliably tracks trends in paleo-production. Journal of Paleolimnology 56:253–265.
Smol, J.P. 2010. The power of the past: Using sediments to track the effects of multiple stressors on lake ecosystems. Freshwater Biology 55:43-59.
Wolfe AP, Vinebrooke RD, Michelutti N, Rivard B, Das B (2006) Experimental calibration of lake-sediment spectral reflectance to chlorophyll a concentrations: methodology and paleolimnological validation. J Paleolimnol 36: 91-100.
Zeeb B, and Smol JP. 2001. Chrysophyte scales and cysts. In: Tracking Environmental Change in Lake Sediments. Kluwer Academic Publishers, Dordrecht

|