University Animal Care Committee Standard Operating Procedure
Document No: 7.20
Subject: Manual Handling and Restraint of Mice
Date Issued: November 14, 2013
Revision: 3
Location: Queen’s University
Responsibility: Principal Investigators (PI), Research Staff, Veterinary Staff
Purpose: The purpose of this Standard Operating Procedure (SOP) is to describe commonly-used handling and restraint techniques for mice.
1. Introduction and Definitions:
The correct application of handling and manual restraint of mice is necessary in order to avoid and/or limit pain and distress. Handling refers to the initial and ongoing contact with the rodent to remove them from their home cage. Handling limits the rodent’s movement to allow for limited clinical assessments, cage transfer, and the application of manual restraint. Manual restraint techniques further limit the movement of the rodent and are valuable tools for competently performing several standard procedures such as blood collection, ear-punching, and clinical assessments. Additionally, restraint aids in administering substances via the most commonly used routes. Limiting the use of anesthesia through proper restraint is of benefit to the animal and researcher.
Handling rodents by the tail had been shown to cause fear, stress, and pain. Additionally, tail handling can lead to injury, especially in heavier rodents. When possible, non-aversive handling techniques should be used. These refined methods have been shown to improve the quality of scientific data and enhance interactions been the rodent and handler. Additionally, they reduce the negative effects of restraint. Tunnel handling is recommended for naive, jumpy, or aggressive mice, while the cupped hand technique is recommended for habituated, calm mice. Tunnel handling may be easier for inexperienced handlers.
It is always best practice to handle rodents calmly and quietly. Odours from perfumes and lotions should be avoided.
Abbreviations:
Animal Care Services ACS, Principal Investigator PI, subcutaneous SC, intravenous IV, intraperitoneal IP, intramuscular IM, per os PO, per rectum PR
2. Materials:
- Personal Protective Equipment (gloves, lab coat, mask, cap)
- Cage and wire lid
- Restrainers
- Clear tunnels
3. Handling Procedures:
Tunnel handling:
- Using a restrainer or tube to transport mice may be less stressful for them than picking them up by their tails, particularly if the handler is inexperienced or nervous.
- The tunnel should be positioned in the cage so that one open end is against a cage wall. The mouse can be gently encouraged to enter the tunnel. With hands cupped over each tunnel opening to prevent escape, the tunnel can be lifted out of the cage. Ensure the tunnel remains horizontal to avoid stress or injury.
- To facilitate an exam or procedure, uncover one end of the tunnel and gently tilt the tunnel to encourage the mouse to walk out onto your hand or a clean surface. Avoid manipulating the mouse by the tail.
- To return the mouse to the homecage, gently place the tunnel into the cage and allow the mouse to exit on its own.
- Repeat as needed for remaining mice in the cage.
- Note that the tunnel should remain in the cage rather than introduced at the time of handling. This will allow the mouse time to voluntarily explore the tunnel prior to handling. If a tunnel is not already present in the cage, allow the mice to voluntarily explore it prior to handling. Thoroughly sanitize any tunnel shared between cages to remove debris, contaminants, and odours.
 http://www.sciencemag.org/news/2018/02/are-happy-lab-animals-better-science
http://www.sciencemag.org/news/2018/02/are-happy-lab-animals-better-science
Cupped hand technique:
- Approach the mouse calmly and quietly at the cage level; avoid overhead movements. Start by lowering one hand into the cage, palm up, and with fingers curved to form a cup. Allow the mouse to explore your hand at its own pace. Guide the mouse into the cupped hand by placing the other hand behind the mouse. Once the mouse is in your hand, use the second hand to form a loose two-handed cup around the animal for support. An open, flat palm can also be used once the mouse is habituated.
- Perform this technique over a soft or secure surface.
- This technique requires habituation and an experience handler. Initial habituation sessions should be kept short and mouse behaviour should be monitored for signs of stress.
Tail handling:
Mice can be gently lifted from their cage and moved as required through the use of their tail. Grasp the base of the tail (proximal or upper 1/3) with the thumb and forefinger of one hand, and place the animal onto your free hand, the cuff of a lab coat (conventional holding rooms only), or the wire top of a cage or cage furniture (e.g., shelter). Do not grasp towards the tip of the tail and do not leave the rodent suspended without additional support for longer than is necessary. This can result in pain or injury to the animal (tail slough), or to the handler if the mouse is able to swing around and bite. Maintain a hold on the tail until restraint is achieved to minimize the risk of the animal escaping. Mice cannot be left unattended outside of their cage. Depending on the hygiene level of the room, the animal may need to be humanely euthanized if it escapes from restraint or its home cage and is outside a sanitized area.
 http://www.theodora.com/rodent_laboratory/restraint.html
http://www.theodora.com/rodent_laboratory/restraint.html
- Mice cannot be left unattended outside of their cage. Depending on the hygiene level (health status) of the room, the animal may need to be humanely euthanized if it escapes from restraint or its home cage and is outside a sanitized area. Any animals that come into contact with the floor must be humanely euthanized and should not be returned to their home cage.
4. Restraint Techniques
Scruff Hold
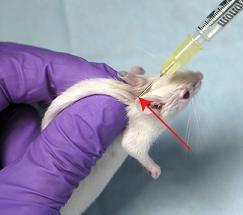
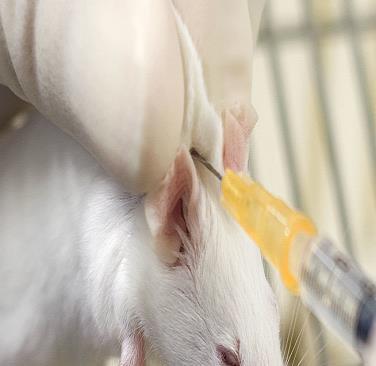
This hold is commonly used for health assessment, ear-punching, oral gavage, submandibular blood collection, genotyping (tail snip) and IP injections.
- Lift the mouse from its home cage to a wire lid, or any other surface that provides traction to the mouse.
- Using your thumb and index finger, scruff the loose skin over the neck, shoulders and back. The mouse’s head should be immobilized, and the front arms slightly splayed. It may help to lightly “slide” finger and thumb along the spine from hind end to shoulders before attempting the scruff.
- Take care not to hold the skin too tightly; this can restrict breathing and potentially suffocate the animal.
- Lift the mouse with the hand performing the scruff hold.
- Secure the tail between the ring or small finger and the palm of the same hand.
- Use the other hand to execute the desired procedure. Adjust the holding angle as required by the specific procedure.

Queen's University Animal Care Services
Tent Hold:
This hold is commonly used for SC injections.
- Lift the mouse from its home cage to a wire lid, or any other surface that provides traction to the mouse.
- Use your thumb and index finger to loosely scruff the skin between the shoulders and form a skin tent.
- Elevate the front legs slightly; this will cause the mouse to grasp the wire lid securely with hind legs for stability.
- Optionally, the mouse may be lifted from its home cage and placed on a wire lid. Tent the loose flank skin and administer the injection. As this technique uses a loose hold, it should only be done by experienced users working with calm strains.
NC3R
| Date | New Version |
|---|---|
| 02/28/2019 | Triennial Review |
| 02/28/2022 | Triennial Review |
| 10/22/2025 | Triennial Review - format update, added tunnel handling and cupped hand handling |
 About Vice-Principal Research
About Vice-Principal Research