University Animal Care Committee Standard Operating Procedure
Document No: 10.10.1
Subject: Tail Vein Blood Collection in Rats
Date Issued: July 7, 2011
Revision: 6
Location: Queen’s University
Responsibility: Principal Investigators, Research Staff, Veterinary Staff
Purpose: The purpose of this Standard Operating Procedure (SOP) is to describe how to properly collect blood from the tail vein.
Abbreviations:
Animal Care Services ACS, Principal Investigator PI, subcutaneous SC, intravenous IV, intraperitoneal IP, intramuscular IM, per os PO, per rectum PR
1. Introduction and Definitions:
Use the following table to ascertain the most appropriate site for blood collection based on the volume required.
| Site | Tail Vein | Saphenous | Cardiac Puncture | Jugular |
|---|---|---|---|---|
| Multiple sampling | Yes | Yes | No | No |
| Volume | 0.05 - 0.1 ml/site |
0.1-0.3 ml | 1.0-3.0 ml | 1.0 ml |
| Gauge (maximum) |
23 | 25 (23) | 23 | 25 (23) |
The following are “good practice” guidelines recommended for blood collection volumes Collection sites and needle gauges.
- The Circulating Blood Volume (CBV) of an adult rat is ~64mL/kg (0.064mL/g).
- 1% (maximum) of the CBV can be collected every 24 hours.
- 7.5% (maximum) of the CBV can be collected in a single collection, once a week.
- 10% (maximum) of the CBV can be collected in a single collection every 2 weeks.
- 15% (maximum) of the CBV can be collected in a single collection every 4 weeks.
To calculate blood collection volumes:
- Body weight x Circulating Blood Volume = Total Blood Volume (TBV)
- TBV x % blood sample required = acceptable volume to be collected (i.e. 100g x 0.064mL/g = 6.4mL/g then 6.4 x 0.075 = 0.5mL is the max accepted volume)
| Body Weight (g) | Total Circulating Blood Volume (ml/g) | Acceptable volume for collection μl (mL) | ||
|---|---|---|---|---|
| 7.5% Single collection/ 1 week | 10% single collection/ 2 weeks | 15% single collection/ 4 weeks | ||
| 100 | 6.4 | 500 (0.5 ml) | 600 (0.6 ml) | 900 (0.9 ml) |
| 150 | 9.6 | 700 (0.7 ml) | 900 (0.9 ml) | 1400 (1.4 ml) |
| 200 | 12.8 | 900 (0.9 ml) | 1200 (1.2 ml) | 1900 (1.9 ml) |
| 250 | 16 | 1200 (1.2 ml) | 1600 (1.6 ml) | 2400 (2.4 ml) |
| 300 | 19.2 | 1400 (1.4 ml) | 1900 (1.9 ml) | 2800 (2.8 ml) |
| 350 | 22.4 | 1600 (1.6 ml) | 2200 (2.2 ml) | 3300 (3.3 ml) |
| 400 | 25.6 | 1900 (1.9 ml) | 2500 (2.5 ml) | 3800 (3.8 ml) |
| 450 | 28.8 | 2100 (2.1 ml) | 2800 (2.8 ml) | 4300 (4.3 ml) |
| 500 | 32 | 2400 (2.4 ml) | 3200 (3.2 ml) | 4800 (4.8 ml) |
When collecting blood it is very important that the handler is able to recognize signs of shock and anemia. The combined effect of sample volume and sample frequency without appropriate fluid replacement can cause an animal to go into hypovolaemic shock or anemia.
- Signs of hypovolemic shock include a fast and thready pulse, pale dry mucous membranes, cold skin and extremities, restlessness, hyperventilation, and a sub-normal body temperature.
- Signs of anemia include pale mucous membranes of the conjunctiva or inside the mouth, pale tongue, gums, ears or footpads (non-pigmented animals), intolerance to exercise and increased respiratory rate at rest with severe anemia.
- Packed cell volume, haemoglobin level, red blood cell and reticulocyte counts should be monitored throughout the series of bleeds using the results from the first sample from each animal as the baseline for the animal.
- If volumes larger than 10% are collected, replace volumes by 3-4 times the blood volume collected with warmed (30-39 degrees) isotonic fluids.
2. Materials:
- Heat lamp
- Restrainers as required
- Sterile syringes (1 – 3 ml)
- Sterile needles (multiple sizes ranging from 23-30g)
- Sterile gauze
- Sterile scalpel blades
- Alcohol swabs
- Petroleum jelly
- Collection tubes
- Warm water and disinfecting soap
- Warmed isotonic fluids such as Lactated Ringers or 0.9% NaCl
3. Procedures:
- Only University Animal Care Committee (UACC) approved blood collection techniques can be performed.
- The least volume required should be collected at all times.
- All collections should be performed by trained and competent individuals.
- The smallest needle size that complements collection location without causing hemolysis should be used.
- Each and every animal requires a new sterile syringe and a new sterile needle/lancet.
- Only three attempts per site should be practiced. If unsuccessful, allow another (trained and competent) person to collect the sample.
- Apply pressure with gauze until hemostasis occurs.
Tail Vein (intravenous)
- Each and every animal requires a new sterile syringe and a new sterile needle/lancet.
- Prepare your sample tubes and have them readily available.
- Release the vacuum on the syringe prior to inserting into vessel.
- Remove the animal from the cage and anesthetize or restrain securely.
- To cause vasodilation, focus the heat lamp on the animal’s tail for 5- 10 seconds, or alternatively, warm the animal by placing a heat lamp at a safe distance above the cage for approximately 5 min.
- Ensure the tail is accessible and the animal is comfortable.
- Gently scrub the tail with surgical soap.
- Identify the lateral tail veins. These are used for blood collection.
- If used, apply EMLA cream to the intended site and wait 15min for it to take effect. Hold the distal portion of the tail straight.
- Swab the tail with alcohol. Using the needle, puncture the vein in an upward motion as if the needle is following the path of the vessel.
- Withdraw the needle and collect the drops of blood.
- For serial collections, a small nick in the tip of the tail using a sharp, sterile scalpel blade is permitted. This technique works well for clot dislodgment and repeated sampling. Hemostasis must be ensured; a cautery pen may be used post-collection, if needed.
- For the collection of larger volumes, a needle and syringe or a butterfly can be used. Pull back on the plunger slowly so that the vessel doesn’t collapse, and obtain desired volume.
- Apply pressure to the site with gauze until hemostasis occurs.
- Return the animal to the cage and monitor for any signs of distress.
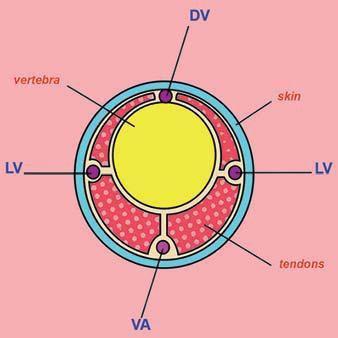
Diagram of a transverse sectional view of a rat tail showing the lateral veins (LV) and ventral artery (VA).
(Modified image reprinted from The Laboratory Rat, G.J Krinke (Ed.), pp. 491, Copyright 2000)
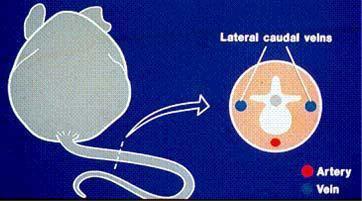
Diagram of a transverse sectional view of mouse tail. dorsal vein (DV), Kathryn Flynn, NIH - DVR - SoBran
- Diehl, K.-H. et al., “A Good Practice Guide to the Administration of Substances and Removal of Blood, Including Routes and Volumes”, J. Appl. Toxicol., 21, 15–23 (2001)
- Wolfensohn, S., Lloyd, M. 2nd Edition, Blackwell Science Ltd. 1998.
- Guidelines for survival bleeding of mice and rats; NIH: http://oacu.od.nih.gov/ARAC/Bleeding.pdf
- Guide to the Care and Use of Experimental Animals, Vol. 1 (2nd ed), Canadian Council on Animal Care, Canada, 1993:
https://ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf - The National Centre for the Replacement, Refinement and Reduction of Animals in Research (NC3R’s) – Blood Sampling Microsite. https://www.nc3rs.org.uk/3rs-resources/blood-sampling
| Date | New Version |
|---|---|
| 09/22/2015 | Triennial review |
| 01/25/2018 | Triennial review |
| 02/28/2019 | Updated SOP |
| 02/28/2022 | Triennial review |
| 07/18/2022 | Original SOP separated into different blood collections SOPs |
 About Vice-Principal Research
About Vice-Principal Research