To access full-sized versions of abstract posters, right-click the image and open in a new tab to view and zoom in.
 Rigid Kelcogel CG-LA Hydrogel Gellan Gum Used As a Vehicle for Surfactants and Chelating Agents in the Cleaning of Sensitive Acrylic Paint Surfaces
Rigid Kelcogel CG-LA Hydrogel Gellan Gum Used As a Vehicle for Surfactants and Chelating Agents in the Cleaning of Sensitive Acrylic Paint Surfaces
Maryse Bonaldo
Wet conservation treatments have proved, under close analysis, to be problematic for sensitive acrylic paint surfaces. Swelling of the paint layers, extraction of paint components that have migrated to the surface of the paint film, and loss of pigment particles are among the most recurrent challenges encountered by conservators cleaning such surfaces. This research will attempt to demonstrate that rigid Kelcogel gellan gum can be an efficient vehicle for surfactants and chelating agents, while providing an adequate and safe alternative to traditional aqueous treatments for cleaning sensitive acrylic paint film. As part of the experimental procedure, three ECOSURF™ EH surfactants and ethylenediaminetetraacetic (EDTA) acid will be added to the gellan gum to create cleaning systems. The capillary action of these systems is expected to provide a controlled diffusion of aqueous solution into the paint film, which should reduce the damage normally associated with the use of water. To maximise the efficacy of the systems and to ensure a safer solution for the paint film, the pH and conductivity will be adjusted with citric acid and triethanolamine (TEA). Cast samples of burnt umber and ultramarine blue acrylic paint from three different commercial brands, as well as a canvas board covered with a coat of standard dirt will be used for tests and analyses. The effects of the cleaning treatments on the paint films, including morphology changes on the surface, will be assessed with stereomicroscopy and scanning electron microscopy-energy dispersion spectroscopy (SEM/EDS). The relative cleaning efficacy of the systems and the presence of cleaning residues left on the surface will be established using Fourier transform infrared (FTIR) spectroscopy. The components that might have been extracted from the paint film by the gellan gum will be identified with gas chromatography-mass spectrometry (GC/MS), and the possible loss of pigments or change in the surface will be measured using a spectrophotometer and a glossmeter. Residue clearing will be considered because of its importance in surface cleaning.
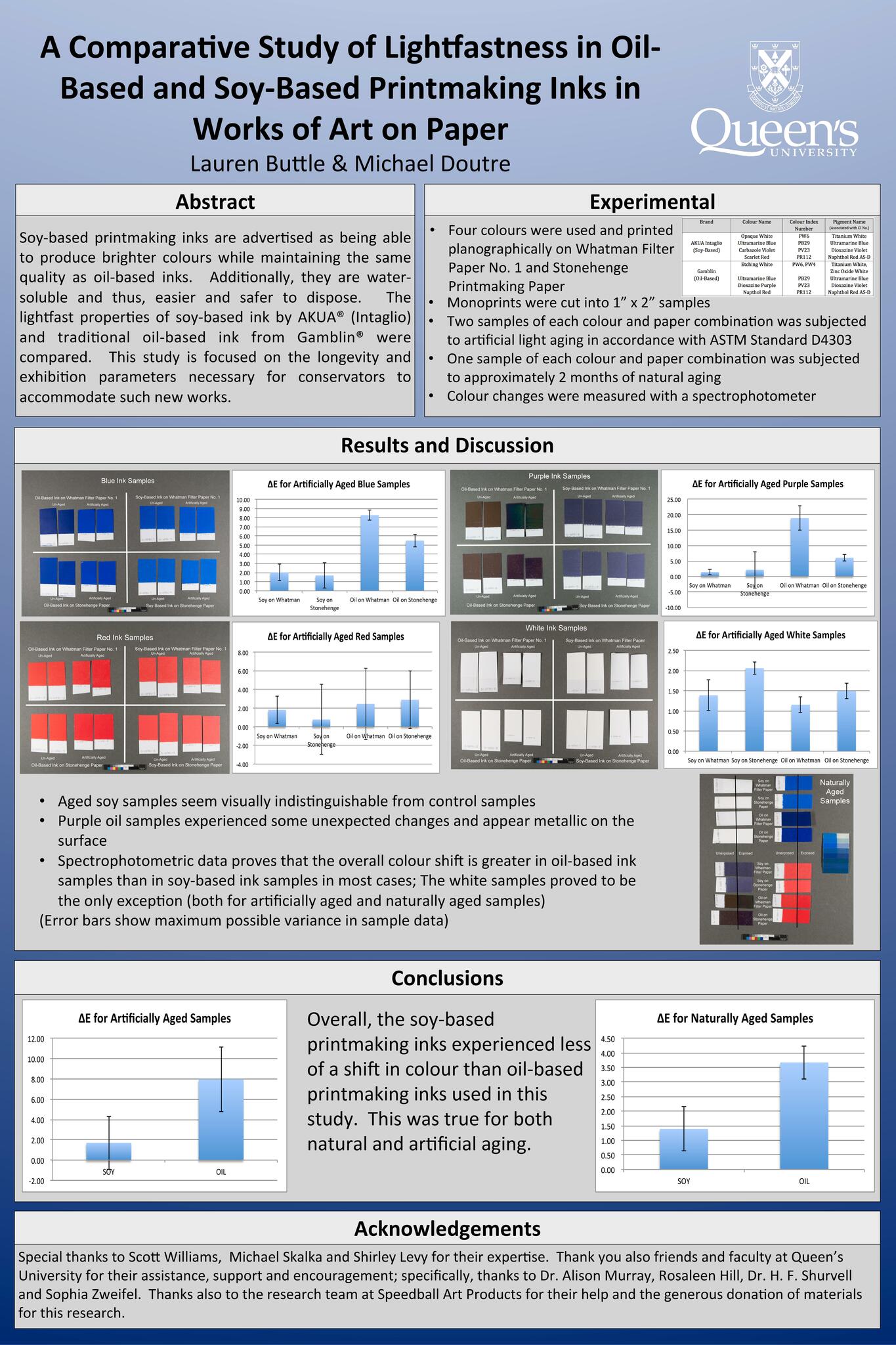 A Comparative Study of Lightfastness of Oil-Based and Soy-Based Printmaking Inks in Works of Art on Paper
A Comparative Study of Lightfastness of Oil-Based and Soy-Based Printmaking Inks in Works of Art on Paper
Lauren Buttle
The purpose of this study is to compare the lightfast properties of soy-based ink in works of art on paper with the lightfast properties of traditional oil-based ink. This study uses commercial products supplied by AKUA® (Intaglio) and Gamblin® and will involve spectrophotometer measurements of four different pigment samples of each type of ink. These samples will be made on Arches printmaking paper and on Whatman® Filter paper in an effort both to replicate real-life circumstances and isolate any optical changes caused by additives in the paper substrate. The samples will be measured before and after natural and artificial light aging. Fourier transform infrared (FTIR) spectroscopy will monitor the chemical shifts in the material.
 Investigation of Sodium Dodecyl Sulfate and Hostacor IT as Flash Rust Inhibitors for Rinsing Archaeological Iron
Investigation of Sodium Dodecyl Sulfate and Hostacor IT as Flash Rust Inhibitors for Rinsing Archaeological Iron
Megan Doxsey-Whitfield
Flash rusting is a common problem for conservators treating wet archaeological iron. After a desalination treatment, the iron object must be rinsed, which typically results in flash rusting. Corrosion inhibitors are not commonly used to prevent this, but could be an effective addition to the treatment procedure. Sodium dodecyl sulfate (SDS) is the main ingredient in Orvus WA paste, an anionic surfactant cleaner commonly found in conservation laboratories. SDS has been found to be an effective anodic corrosion inhibitor for copper and nickel in acidic solutions. This study will investigate if SDS is a good corrosion inhibitor for iron by comparison with Hostacor IT, a product known to be effective. SDS is being tested as an alternative to Hostacor IT because it is more cost effective, more readily available, and less hazardous to the environment. Hostacor IT has a history of use in industrial practices. Additionally, it has been tested and used as a corrosion inhibitor in conservation for composite iron and wood objects being treated in polyethylene glycol. This experiment will evaluate the effectiveness of three concentrations of SDS in water at preventing flash rusting on coupons made from cold-rolled steel. The results will be compared with coupons in a solution of Hostacor IT 1% (v/v) in water and a control solution of water with no corrosion inhibitor. The metal coupons will be evaluated visually, and by using a spectrophotometer to determine the presence of flash rusting. The flash rust on the control will be analyzed using X-ray diffraction. It is expected that Hostacor IT and SDS will both prevent the metal coupons from flash rusting. If SDS proves as effective as Hostacor IT, it may be a suitable additive to the rinse stage of cleaning archaeological iron.
 Examining the Effect of Relative Humidity on Mammoth Molars
Examining the Effect of Relative Humidity on Mammoth Molars
Daniel Doyle
Museum and natural history collections are very sensitive to fluctuations in relative humidity. Because of a high degree of structural anisotropy, mammoth molars are potentially damaged by such fluctuations. Upon excavation, mammoth molars often undergo rapid acclimatization to the ambient RH, with resulting delamination and cracking. Research conducted on mammoth molars by Samantha Fisher in the Art Conservation Program at Queen’s in 2014 found that cracks widen and extend after exposure to 75%RH, that cracks continue to extend after the drop to 35%RH, that cement adsorbs and desorbs faster than enamel, and that Fourier transform infrared spectroscopy shows the presence of hydroxyapatite and water with some carbonate and fluoride substitutions in molars. As the molars used had already undergone humidity cycling, no significant changes to cracks were observed during the experiment. To further understand the changes occurring during significant drops to low RH, as during the initial excavation and drying of the molars, a single cycle will be undertaken between 11%RH using a saturated lithium chloride solution and 75%RH using saturated sodium chloride solution. Weight changes indicating the adsorption and desorption of water from the teeth will be measured to better understand the internal moisture change, and to identify when the molars have reached equilibrium. The resulting dimensional changes and crack propagation will be measured using ImageJ analysis software and physically with calipers. This is only one of a very limited number of studies into the effects of relative humidity on natural history collections. A survey of current storage conditions for natural history collections may be undertaken, to identify current practices and possibly propose preventative steps.
 An Assessment of the Effects of Ammonium Citrate on Paper
An Assessment of the Effects of Ammonium Citrate on Paper
Laura Hashimoto
Ammonium citrate has been used in its dibasic (ACD) and tribasic (TAC) forms in art conservation, primarily for the surface cleaning of paintings and in the removal of metallic staining on objects such as marble and leather. This chelating agent is easily adjustable for neutral and basic pH levels, recommending it for use in paper conservation to solubilize and remove acidic soiling and staining. Based on Antoinette Dwan’s techniques and studies on the use of ammonium citrate for the removal of these constituents, the goal of this research is to determine what, if any, physical and chemical effects occur after paper samples are treated with the prepared solutions followed by artificial aging. Whatman No. 40 paper, rag paper that has been dyed with Prussian blue and naturally aged, and a naturally aged newsprint paper will be immersed in 1% and 3% w/v ammonium citrate solutions adjusted to a pH of 7 with ammonium hydroxide. Thermal accelerated aging will then be carried out. The papers will be assessed according to colour change using a spectrophotometer, pH change using a pH meter, and changes in mechanical strength using zero-span tensile testing. The research will qualitatively compare the efficacy of the ammonium citrate in established aqueous deacidification and alkalization washing techniques. This preliminary testing will be focused on determining if ammonium citrate is a viable option as a supplement to aqueous treatments, or as an alternative to a variety of treatments including solvents and bleaching.
 The Use of Silicone-based Microemulsions in Paper Conservation: Investigating Residue and Discoloration
The Use of Silicone-based Microemulsions in Paper Conservation: Investigating Residue and Discoloration
Natasa Krsmanovic
The goal of this research is to determine if silicone-based microemulsions leave behind a residue on paper substrates. Three paper substrates will be used to test the removability of a microemulsion on paper and the appropriateness of the silicone-solvent on different manufactures of paper. A Whatman No. 1 Filter Paper, a heavily sized paper, and a paper with optical brighteners will be used. A microemulsion consisting of Cyclomethicone D4, Ecosurf EH-3, and water will be applied to the papers and cleared using hexamethyldisiloxane. One set of samples will have a barrier between the microemulsion and paper, while another will be applied directly to the paper substrates. Another set of samples will be untouched, acting as the control, while a third set will be immersed in a solvent bath of Cyclomethicone D4. The results will be analyzed for color change and the presence of residues using spectrophotometry and scanning electron microscopy. It is hypothesized that the silicone-based microemulsion will leave some residue on or within the paper substrates even after clearance. The method of applying the gel and clearing the emulsion will probably affect the amount of residue within the papers. The low viscosity of the silicone-based microemulsions and the low surface tension of the solvents raise some concerns about the level of control during “wetting out” of the paper substrates. This issue will be monitored and documented as qualitative analysis during the study.
 Gatorfoam as a Physical Support for Paintings Anticipating Handling
Gatorfoam as a Physical Support for Paintings Anticipating Handling
Bethany Jo Mikelait
Gatorfoam is a board of polystyrene foam laminated between two panels of proprietary resin-coated wood fibre veneer. The purpose of this study is to investigate the function and effectiveness of Gatorfoam in paintings conservation as a rigid, direct-contact support placed between the stretcher and the canvas. This material in theory would provide a stable physical support for a fragile painting by dampening vibrations due to handling, reducing the effects of shock, and minimizing the effects of varying humidity and temperature. Research on this material and application will proceed with mechanical testing, to evaluate the physical stability imparted to a painting exposed to such shock as accidental dropping during in-house handling. Surrogate paintings with brittle gesso coatings will be subjected to corner, edge, and flat drops using a commercial drop-tester. Fragility ratings will be assigned to facilitate the comparison of these very uniform samples with and without Gatorfoam supports, and allow a measure of quantification in the degree of improvement the Gatorfoam treatment provides. Additional surrogates will be prepared with a Coroplast backing board and drop-tested to assess whether the more invasive Gatorfoam treatment provides sufficiently better protection to warrant the higher risk involved in removing the painting from its stretcher. A percentage will be assigned to the degree of improvement. Conservators will be able to make a better-informed decision when weighing the risks related to removing a painting from its stretcher for the purposes of structural and mechanical stabilization.
 The Effect of Cyclododecane on Aged Acrylic Paint Films
The Effect of Cyclododecane on Aged Acrylic Paint Films
Marie-Hélène Nadeau
This project will study the use of cyclododecane as a tool for the treatment of acrylic paint films. Cyclododecane (C12H24) is a stable molecule that sublimates and does not leave residues. It is a safe and widely used product for paper and archeological materials, but it has not been extensively used by painting conservators. Used as a temporary consolidant or a protection layer, cyclododecane would allow conservators to perform treatments such as tear repairs, flattening and mechanical removal more safely. Of concern is the possibility that cyclododecane might leave residues in the paint film and cause products to migrate to the paint surface. Cyclododecane will be tested on three pigments from two brands of acrylic paints. The samples used have been naturally aged to evaluate how cyclododecane affects an aged paint-film. Gloss, colour and surface tension measurements will be performed on cyclododecane-coated and uncoated samples. In addition, Fourier transform-infrared spectroscopy (FT-IR) and scanning electron microscope (SEM) analyses will be performed in order to evaluate possible migrating products and the presence of residues. It is expected that cyclododecane will also be safe for use on paint films.
 Fill Materials for Cast Acrylite® Used in Face-Mounted Photographs: Scratch-Repair, Methodology, and Accelerated Aging
Fill Materials for Cast Acrylite® Used in Face-Mounted Photographs: Scratch-Repair, Methodology, and Accelerated Aging
Kaslyne R. O’Connor
A primary conservation issue for the sustained use of face-mounted photographs is the long-term stability and inherent susceptibility of the acrylic sheet surface to abrasions of the acrylic sheet surface. Scratches disfigure the entire surface of the poly(methyl methacrylate) sheet by changing the surface topography and distracting the viewer from the photograph. Scratches on acrylic sheet surfaces can be the result of improper handling, storage, and routine cleaning, and can range in size from micrometers to centimeters in size. The care, storage, and conservation of these acrylic face-mounted photographs have become growing subjects of debate and preservation experiments among the conservation community. The primary goal of this study is to determine the quality of scratch repairs for acrylic sheeting and their suitability for the conservation and preservation of face-mounted photographs. Mechanical scratches will be made on Acrylite ® sheets samples and filled manually with three selected materials (an acrylic copolymer, an ultra-violet curing adhesive, and two-part epoxy) in one and two layers, each displaying effective refractive indices, low viscosities, resistance to yellowing and favourable working times. Samples will be subjected to accelerated thermal and light aging to detect any negative effects on the poly(methyl methacrylate) caused by the application and manipulation of these scratch fillers. Materials used in successful repairs will undergo analytical comparative tests with a spectrophotometer and glossmeter for colour change or surface distortion, as well as having visual comparisons of ensuing scratch visibility. The success or failure of the fills will be evaluated according to their ability to reduce the appearance of scratches without negatively impacting the finishing surface.
 Thataway Again: A Case Study of Weathering Steel Corrosion
Thataway Again: A Case Study of Weathering Steel Corrosion
Carolyn Savage
Canadian contemporary sculptures are exposed to extreme temperature fluctuations, air pollution, rain and vandalism, causing deterioration of the paint layer through its failure or biological attack. The outdoor installation Thataway Again by Henry Saxe is a painted steel sculpture made in 1979, acquired in 1982 and located on the south lawn of Harrison-LeCaine Hall, Bader Lane, Queen’s University. An investigation of Thataway Again revealed that the sculpture is exhibiting surface corrosion and biological activity in some areas. In order to complete a comprehensive treatment proposal, information will need to be obtained on the type of paint used by Saxe in his outdoor installations, along with his application methods, potentially through access to the Agnes Etherington dossier or communication with the artist or foundation. The identity of the paint medium will be confirmed using micro-destructive Fourier transform infrared spectroscopy (FTIR) and pyrolysis-gas chromatography-mass spectroscopy (Py-GC-MS). Further identification of the steel and corrosion products will involve non-invasive x-ray fluorescence (XRF) to obtain elemental information and micro-destructive x-ray diffraction (XRD) to identify the crystalline materials in the corrosion products. Treatments of outdoor painted steel involve removal of the paint layers and corroded surfaces to prepare the metal surface for the application of another paint system. Sometimes protective coatings are applied. Once the paint type and application method for the treatment have been determined, steel coupons will be painted with a similar paint system and application method. The efficacy of the anti-graffiti protective coating PSS 20 to protect painted outdoor steel sculpture will be determined. This coating has been described by the manufacturer as easily reversible, clear, non-toxic, biodegradable, environmentally friendly, and compatible with most surfaces, including painted surfaces. The coupons will undergo artificial light aging to simulate three- and ten-year intervals and will be tested for colour change and gloss change. These results will establish the long-term colour-fading properties of protective anti-graffiti coatings and demonstrate the value of treating an outdoor painted steel sculpture in an urban environment.
 Exploring the Role of the Substrate in the Fading and Reversion Behaviour of Prussian Blue
Exploring the Role of the Substrate in the Fading and Reversion Behaviour of Prussian Blue
Sophia Zweifel
The pigment Prussian blue (ferric ferrocyanide) is known to exhibit photochromic behaviour due to its isotropic structure. Under prolonged exposure to light or in the absence of oxygen, Prussian blue will fade to Prussian white as its ferric iron is reduced to the lower oxidation state of ferrous iron. The compound will revert back to Prussian blue when re-exposed to dark, ambient conditions. While the fading and reversion mechanism of Prussian blue is largely understood, these behaviors are complicated by environmental conditions, by the presence of production additives, and by the substrate upon which it is suffused. For example, it is not known whether a proteinaceous substrate might influence the reduction-oxidation reaction of Prussian blue differently than would a cellulosic substrate. This is particularly important in the case of Prussian blue dye, which was in common use during the nineteenth century. The role of the substrate in the fading and reversion of Prussian blue dye will be analyzed by measuring the degree and rate of Prussian blue fading and reversion on different substrates (particularly cotton and silk). One set of samples will be exposed to light under ambient conditions as well as under anoxia. Colour change and reaction rate will be measured using a portable microfade tester. Once fading has occurred, the portable microfade lamp will be turned off and the device's spectrometer will be left focused on the spot in order to measure the reversion rate of the sample. A second set of samples will be exposed to daylight under regular and anoxic conditions over a longer period of time in a well-lit window. The colour change of these samples will be measured by a portable spectrophotometer. Additionally, samples will be analysed by Fourier transform infrared spectroscopy in order to demonstrate the chemical change responsible for the fading and reversion of Prussian blue dye.